Cerba Research offers global NGS capabilities dedicated to your trial.
Cerba Research and Healthcare has been involved in human genetic testing since 1992.
Thanks to this experience, we can collaborate with the life science community to provide the most suitable genomic service and enhance the power of your genetic material.
We offer global NGS capabilities with numerous comprehensive panels and solutions for your trial in oncology, infectious diseases, rare diseases, cell and gene therapies and more.
Our capacity for high throughput, with the ability to sequence more than 1,000 whole human genomes in as little as 10-15 days, provides the information needed to bring targeted medicines to patients faster.
Targeting genes of interest is critical to ensure an accurate outcome for your trial. Our scientific team is dedicated to supporting the customization of your broad panel NGS assays.

Create Your Project With Customizable Panels In Mind

From sample collection to reporting, Cerba Research offers an end-to-end service dedicated to you Next Generation Sequencing needs.
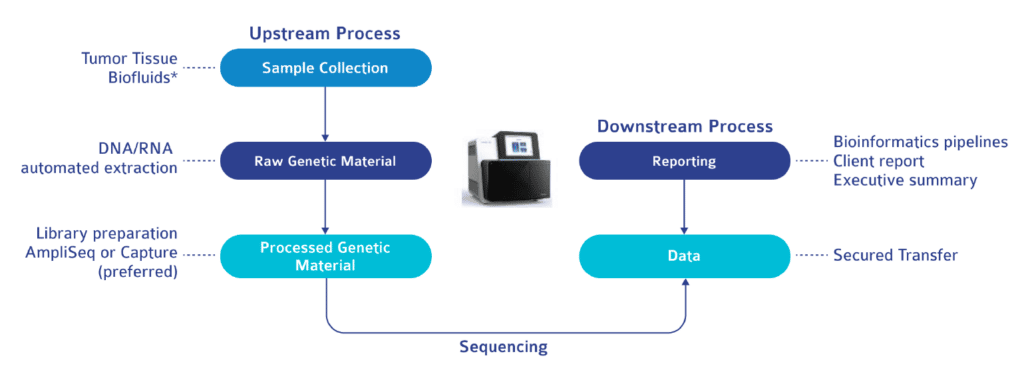
Allow your trial to use a fully automated FDA and CE-IVD-approved platform
Benefit from our biomarker testing offerings. Our scientific team is happy to help you with your custom assay and panel requirements.
- MiSeqDX instruments (FDA regulated and CE-IVD marked)
- NextSeq 500
- NextSeq2000
- NextSeq550Dx (FDA regulated and CE-IVD marked)
- Cobas 4800
- BioRad QX200+ AutoDG
- Applied Biosystem 3500

Reach out to our genomics team and see how we can help advance your clinical research with Next Generation Sequencing
Contact Us
