What Is A Solid Tumor?
A solid tumor is an abnormal tissue mass that usually originates from an organ. Solid tumors are either benign (non-cancerous) or malignant (cancerous). Different types of solid tumors are named based on the cell type that they originate from (1). Examples of solid tumors are carcinomas, adenocarcinomas and sarcomas, and the most diagnosed cancers are lung, breast, prostate, and colorectal cancer (2).
With increased complexity in solid tumor protocol design, endpoints, emerging biomarkers, and time sensitivity due to end-of-life clinical trial patients (3), Cerba Research provides an adaptive oncology team with access to the latest science and technology and offers a wide range of integrated specialty and safety testing for solid tumor clinical trials. Get in touch to learn more.
Cerba Research Solid Tumor Services
Solid tumor research and development often face significant challenges, and complex clinical trials require a laboratory partner who provides services throughout the development spectrum. Cerba Research understands the complexities of cancer research, its associated treatment guidelines (4), and biomarkers used for various study endpoints. Cerba Research offers a wide range of services that can specifically support solid tumor research in this new era of precision medicine. We also offer end-to-end oncology solutions from discovery to post-market authorization for your global / regional trials.
In the past 5 years, Cerba Research performed many early-phase solid tumor projects. Most of our first-in-human (FIH) studies include dose escalation / expansion studies with patient populations suffering from advanced or metastatic solid tumors. Cerba Research also has experience with breast cancer, prostate cancer, non-small cell lung cancer (NSCLC), and colorectal cancer (CRC), among other solid indications. Notably, the laboratory has played a pivotal role in the approval of innovative drugs for indications like breast cancer, NSCLC, and high-risk neuroblastoma.
Our team is experienced in many innovative techniques. We have a global network of laboratories that offers a wide range of capabilities, ranging from safety testing (also known as routine testing) to NGS broad-panel assays, ctDNA panels, immunohistochemistry (IHC), flow cytometry (FCM), immunoassays, immunogenicity, qPCR, ddPCR, NanoString®, and more. We also facilitate state-of-the-art technologies, subject matter expertise, specialized logistics, and operations to accelerate your oncology research and development. Interested in artificial intelligence (AI) image analysis for your lung cancer trial? Check out our resources here.
Remember that if we don’t have it, we can customize it. Thanks to our expertise in assay development, validation (5), and customization capabilities, we have garnered ~16,000 patients screened and 12,710+ randomized within our solid tumor programs since 2018.
We Perform Specialty Testing In ~65% Of Our Solid Tumor Portfolio
We have had 100+ solid tumor trials since 2018 and growing. We perform specialty testing in ~65% of cases within those trials. Specifically, we can perform immunohistochemistry (IHC), flow cytometry (FCM), and/or next-generation sequencing (NGS), amongst other innovative techniques. We can also perform any routine testing (aka safety testing), such as, but not limited to, coagulation, biochemistry, urinalysis, pregnancy test, COVID testing and serology, which are essential for any oncology trial and patient inclusion / exclusion criteria. We can also help design and validate specialty-based assays that account for the complex tumor microenvironment of solid tumors.
Learn More About Our Solid Tumor Drug Development Capabilities
NGS, Oncopanels, broad panels, custom panels
RNAseq
Single-gene
ctDNA-based panels
ddPCR, qPCR
Whole exome / whole genome
HLA typing
TCR / BCR seq
NanoString®
SNP-Array
DNA/RNA extraction
Streck Cell-Free DNA BCT®
PaxGene®, Qiamp kits
Coagulation
Hematology
Biochemistry
Urinalysis
Pregnancy test
COVID test
Serology
Thyroid function
Multiplex cytokine profiling (37-plex)
50+ ligand binding assays
ELISA
ELLA
MSD
ELISpot
PK / ADA /Nab
FCM
Cytek Aurora
Immunophenotyping (including intra-cell markers)
Receptor occupancy
MRD detection
CAR T cell enumeration
CAR T cell phenotyping
Intracellular cytokine detection
PBMC Isolation
BMMC Isolation
Optical genome mapping, our next-generation cytogenetics
PK/ADA/Nab
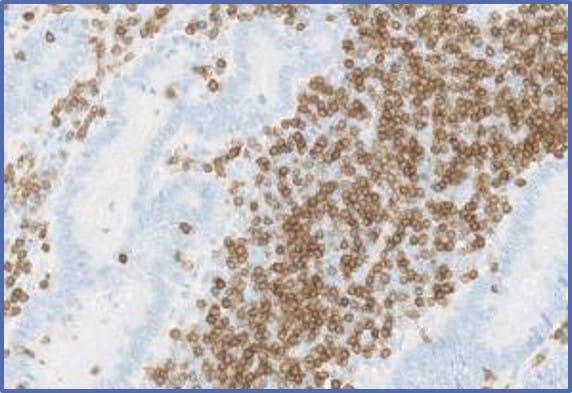
Multiplex / simplex IHC
250+ biomarkers / protocols
Full histopath service
Halo®, Visiopharm®, AIForia®
Board certified pathologists
Large biobank
Strong immuno-oncology simplex & multiplex panels
Spatial analysis of the tumor microenvironment
NanoString® GeoMx, FISH, ISH
End-to-End Services Across Your Trial Continuum
Cerba Research can execute upon every solid tumor trial phase, ranging from discovery / pre-clinical (data not shown) to post-market authorization trials. As such, our most decisive experience in this space is with FIH / phase I and phase II trials, which comprise close to 80% of our solid tumor portfolio. We engage early on in oncology trials during FIH trials, where our sponsors often continue to work with us on full asset programs until registration trials and beyond. This intelligence, along with custom assays and on-target protocol advice, can accelerate your program to market.
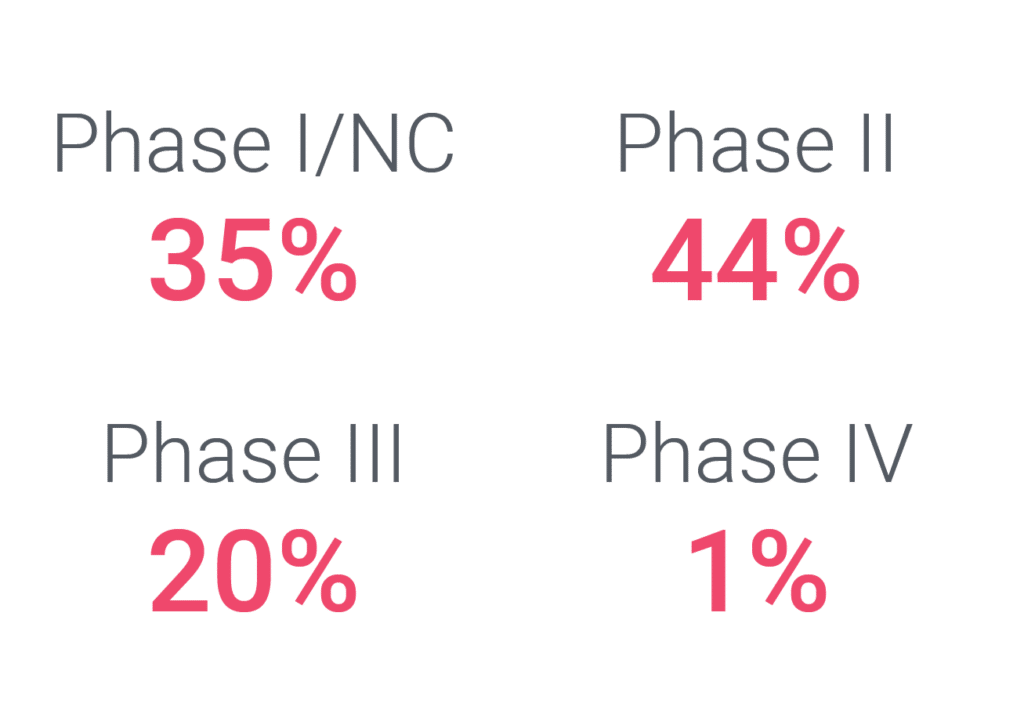
Cerba Research has supported biopharma and pharmaceutical companies in developing oncology therapies. Our heritage in specialty labs and our experience in central lab services enable us to develop research techniques to underpin the next generation of clinical trials. A new kind of research where diagnostics are driven by clinical data and insights, supported by specific therapeutic expertise.
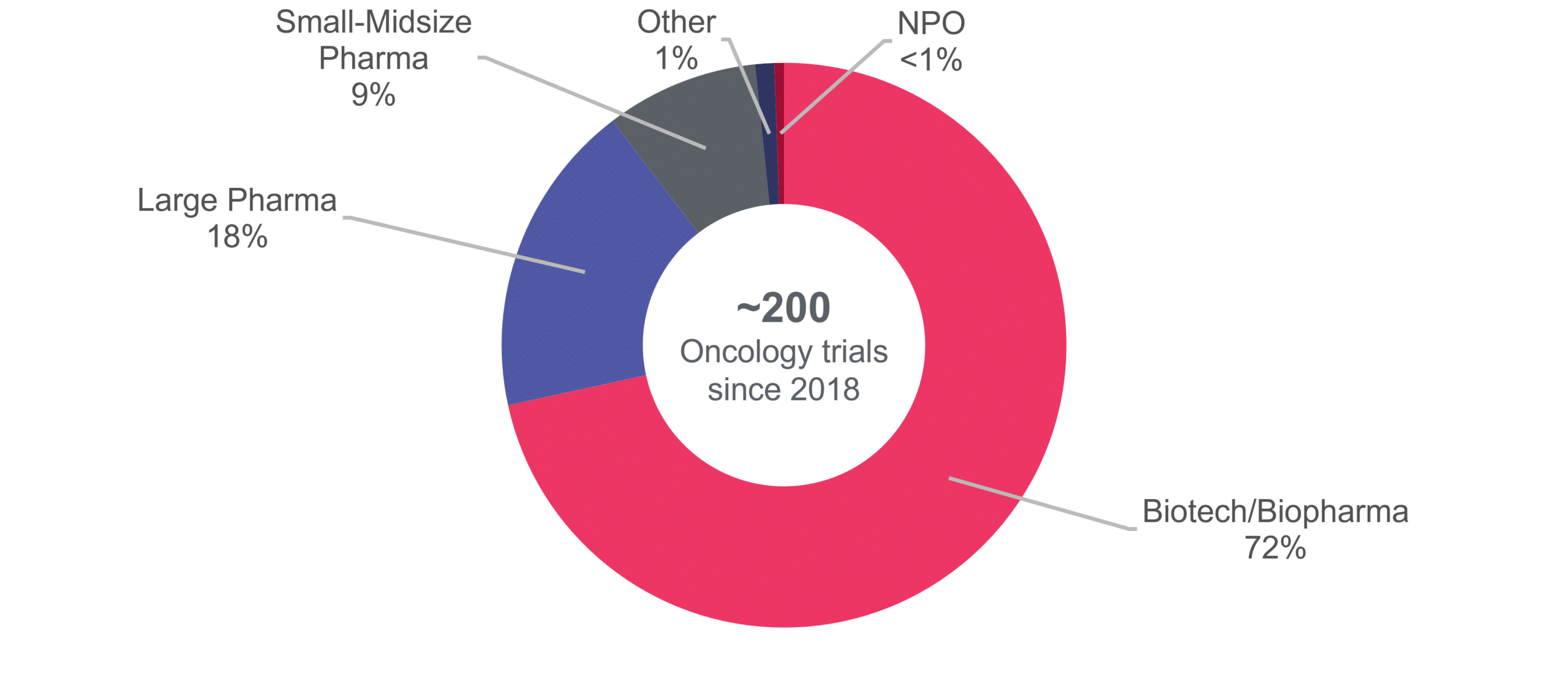
Our Areas Of Expertise In Solid Tumors
We have expertise in various solid tumor indications. Cerba Research participated in the approval of 12 novel therapies, which were approved for indications such as metastatic breast cancer, neuroblastoma, and NSCLC, amongst others. Our therapy class experience is highest with small molecules (50% of our solid tumor trials), followed by monoclonal antibodies (mAbs 27%), antibody-drug conjugates (ADCs 7%), and cell & gene therapy (CGT 5%).
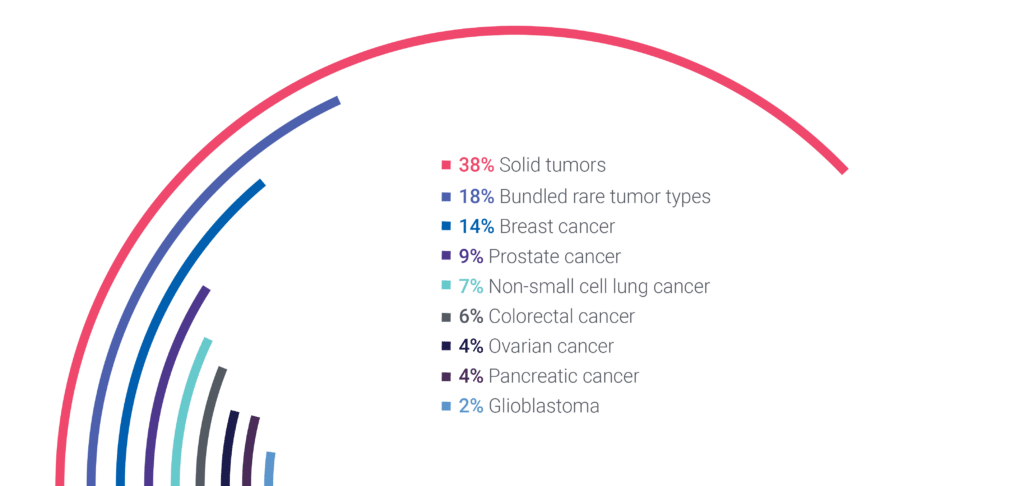
Genetics & Genomics For Solid Tumors
At Cerba Research, our expert team can optimize solid tumor trials due to our extensive experience in genomics and access to a full range of sophisticated instruments. We are also continuously verifying solid tumor international guidelines (4) and adapting our existing panels accordingly.
Cerba OncoSign 600+
Benefit from Cerba OncoSign 600+, our extra-large NGS panel. Our comprehensive solid tumor profiling assay, which is CE-marked, supports the identification of DNA and RNA fusions implicated in various solid tumor types. This comprehensive tumor genomic profiling assay evaluates 638 genes, including 20 fusions, for multiple variant types, including tumor mutational burden (TMB), microsatellite instability (MSI) & homologous recombinant deficiency (HRD). It covers mutations with established, emerging & exploratory value across lung, ovarian, breast, colon, melanoma, bladder, brain, sarcomas, and more. It is performed on formalin-fixed paraffin-embedded (FFPE) samples with at least 20% tumor cellularity, with 2 tubes containing 5 curls of 5 u thickness (1 for DNA & 1 for RNA).

Cerba OncoSign FFPE
Cerba OncoSign FFPE panel covers mutations with established and emerging values across the lung, ovarian, breast, colon, melanoma, bladder, brain, and more. This comprehensive tumor genomic profiling assay evaluates 50 genes, including 17 fusions, for multiple variant types. It is also performed on FFPE for routine practice in parallel with HRD status, which is CE-IVD marked. It is performed on FFPE with 2 tubes containing 5 curls of 5 u thickness (1 for DNA & 1 for RNA).
The gene list: AKT1, ALK, AR, ATRX, BAP1, BRAF, BRCA1/2, CDK4/6, CDKN2A, CTNNB1, EGFR, EIF1AX, ERBB2/3, ESR1, FGFR1/2/3, FOXL2, GNA11, GNAQ, GNAS, H3C2/3, H3F3A/B, HRAS, IDH1/2, KEAP1, KIT, KRAS, MAP2K1, MET, MYD88, NRAS, PDGFRA, PIK3CA, POLD1, POLE, PTEN, RAF1, RB1, RET, SF3B1, STK11, TERT, TP53.
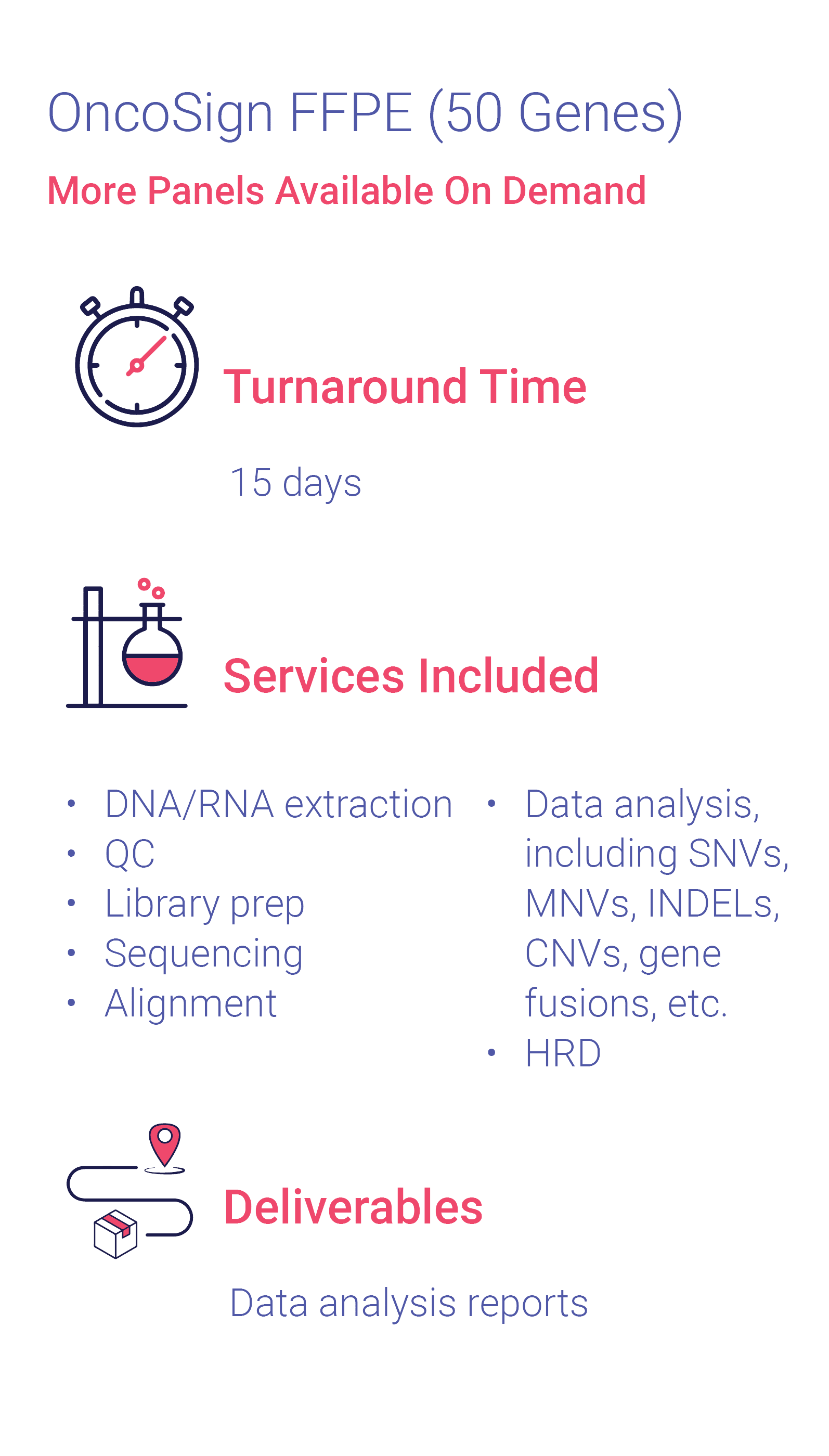
Cerba OncoSign ctDNA
Cerba OncoSign ctDNA panel covers mutations with established and emerging values across the lung, ovarian, breast, colon, melanoma, bladder, and more. This comprehensive tumor genomic profiling assay evaluates 50 genes for multiple variant types for the detection of AKT1, ALK, AR, ATRX, BAP1, BRAF, BRCA1/2, CDK4/6, CDKN2A, CTNNB1, EGFR, EIF1AX, ERBB2/3, ESR1, FGFR1/2/3, FOXL2, GNA11, GNAQ, GNAS, H3C2/3, H3F3A/B, HRAS, IDH1/2, KEAP1, KIT, KRAS, MAP2K1, MET, MYD88, NRAS, PDGFRA, PIK3CA, POLD1, POLE, PTEN, RAF1, RB1, RET, SF3B1, STK11, TERT, TP53. It is performed on liquid biopsies with 2 tubes of 8,5 ml (Streck Cell-Free DNA BCT®).

Immunogenicity In Solid Tumor Trials
Our clients are supported from pre-clinical to post-market authorization with our in-depth biologics and biosimilars experience. We offer long-standing scientific expertise in pharmacokinetics (PK) and immunogenicity (antibody-drug antibodies (ADAs) and neutralizing antibodies (NAbs)) with a standardized or a customized approach as per your vision.
Did you know that Cerba Research Canada was instrumental in the PK and immunogenicity validation and implementation of bevacizumab? A mAb is approved globally for various solid tumors, such as metastatic colorectal cancer, advanced or metastatic non-small cell lung cancer, and recurrent glioblastoma, among other tumor types (6).
Our focus extends from mAbs to CGTs, ADCs, and more. In addition, we are Good Laboratory Practice (GLP), the College of American Pathologists (CAP), and Clinical Laboratory Improvement Amendments (CLIA) accredited for your regulatory requirements.
References
1. National Cancer Institute: NCI dictionary of cancer terms. URL [Definition of solid tumor – NCI Dictionary of Cancer Terms – NCI]
2. NIH Surveillance, Epidemiology, and End Results Program (SEER): Cancer stats facts, common cancer sites. URL [Common Cancer Sites — Cancer Stat Facts].
3. Getz K, Smith Z, Kravet M. Protocol Design and Performance Benchmarks by Phase and by Oncology and Rare Disease Subgroups. Ther Innov Regul Sci. 2023 Jan;57(1):49-56. doi: 10.1007/s43441-022-00438-5. Epub 2022 Aug 12. PMID: 35960455; PMCID: PMC9373886.
4. National Comprehensive Cancer Network®: NCCN guidelines, treatment by cancer type. URL [Treatment by Cancer Type (nccn.org)].
5. Selliah N, Nash V, Eck S, Green C, Oldaker T, Stewart J, Vitaliti A, Litwin V. Flow Cytometry Method Validation Protocols. Curr Protoc. 2023 Aug;3(8):e868. doi: 10.1002/cpz1.868. Erratum in: Curr Protoc. 2024 Jan;4(1):e988. PMID: 37606503.
6. Avastin® (bevacizumab): Highlights of prescribing information. URL [avastin_prescribing.pdf (gene.com)].
Discover Our Expertise In Transforming Research For Your Solid Tumor Treatments

We recommend starting engagement with our scientific team early, such as at the protocol design phase, for optimal results. Reach out to us here.
Contact Us