ASCO 2022 – Standing ovation and hope.
Astra Zeneca and Daiichi Sankyo’s Enhertu is the first targeted treatment prolonging progression-free survival of HER2-low breast cancer patients.
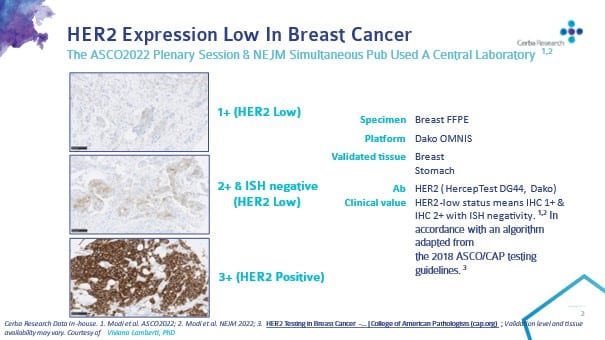
Presented in the @NEJM and at #ASCO2022 (Phase 3 Destiny-Breast04 clinical trial), Enhertu almost doubled median progression-free survival to 10.1 months over chemotherapy (5.4 months) in women with HER2-low, estrogen receptor-positive breast cancer, equivalent to a 49% reduction in the risk of progression. Around half of all breast cancers are considered HER2-low (IHC score 1), meaning that a major life-changing drug is on its way for hundreds of thousands of patients.
Trastuzumab deruxtecan (formerly DS-8201), an antibody–drug conjugate consisting of a humanized anti-HER2 monoclonal antibody linked to a topoisomerase I inhibitor payload through a tetrapeptide-based cleavable linker, has been approved for the treatment of patients with metastatic HER2-positive breast cancer who have received prior anti-Her2 based treatment and relapsed. It binds to and blocks the signaling of the epidermal growth factor reception 2 (Her2).
Once it is bound to Her2, it is internalized and the payload (deruxtecan) interferes with DNA replication during mitosis, ultimately resulting in DNA damage and cell death. A main adverse event caused by Enhertu is interstitial lung disease (ILD), which will need to be carefully monitored in Her2 low breast cancer patients during the administration of this drug.
At Cerba Research, we pride ourselves on providing state-of-the art histopathology services, alongside our global central lab footprint. With a biobank of over 3,000 samples and Her2 as a validated marker, we are ready to take on the challenge to expand our capacity and support the development of more breast cancer drugs that will undoubtedly come to light on the tails of this fantastic new development.”