What Are Metabolic Diseases?
Metabolic diseases are a group of disorders that disturb the normal metabolism activity, which is the process of transforming food into energy at the cellular level. These diseases affect the breakdown of proteins, carbohydrates, lipids, and other elements and can be caused by problems with organs such as the liver or pancreas (1). Some common metabolic disorders include:
- Diabetes: A group of metabolic disorders that affect the body’s ability to use insulin (1).
- Obesity: An excess of body fat that frequently results in impaired health and well-being (1).
- Nonalcoholic steatohepatitis (NASH): A type of nonalcoholic fatty liver disorder (NAFLD), which is a condition in which the liver builds up an excess of fatty deposits. NASH is accompanied by inflammation and may cause liver damage (2).
At Cerba Research, we understand that our greatest strength is our people. Our expert teams around the globe can shape your metabolic disease trial with our wide range of safety, specialty, and logistics solutions. Get in touch to learn more.
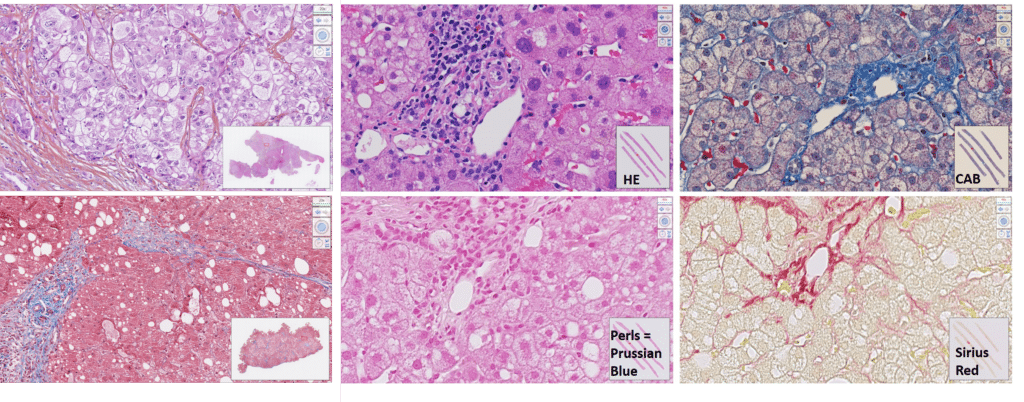
6 Validated Stains, 1 Pathologist Reading Slides
Cerba Research Metabolics Services
Cerba Research is uniquely positioned to support your metabolic trial with a wide range of in-house laboratory solutions depending on your specific indication. Thanks to our expertise in assay development, validation (3), customization capabilities, robust kit building, sample management, and logistics, we have garnered 26,000+ patients screened from 2,100+ clinical trial sites in our metabolics program since 2012.
Metabolic clinical trial designs are nestled into routine testing (also known as safety testing), such as glycemic measurements and liver function tests. A such, Cerba Research offers a wide range of expertise and capabilities that can specifically support your routine tests centrally. Such solutions include:
- Hematology
- Biochemistry
- Coagulation
- HbA1c
- Random plasma glucose
- Fasting plasma glucose
- Urinalysis dipstick
- Urinalysis sediment
- Urine chemistry
- Urine electrolytes
- Microalbumine / creatinine
- Serum pregnancy test
- Serology
- Endocrine tests (TSH, T3, T4)
- And more

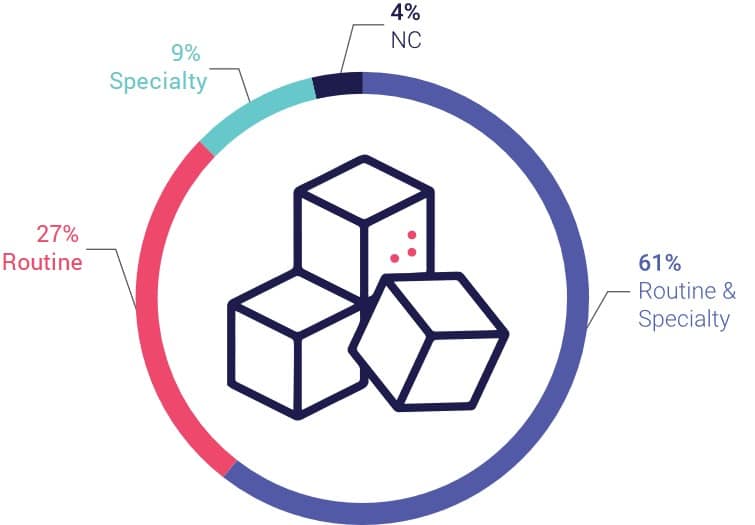
We Perform Routine Testing In ~90% Of Our Metabolics Portfolio
We have had ~60 metabolic trials since 2012 and growing. We perform routine testing centrally in about 90% of cases within those trials. We can perform any routine testing, such as, but not limited to, coagulation, biochemistry, urinalysis, pregnancy test, COVID testing and serology, which are essential for any metabolic disease trial and patient inclusion / exclusion criteria. We can also help design and validate specialty-based assays that measure liver, glycemic and lipid biomarkers.
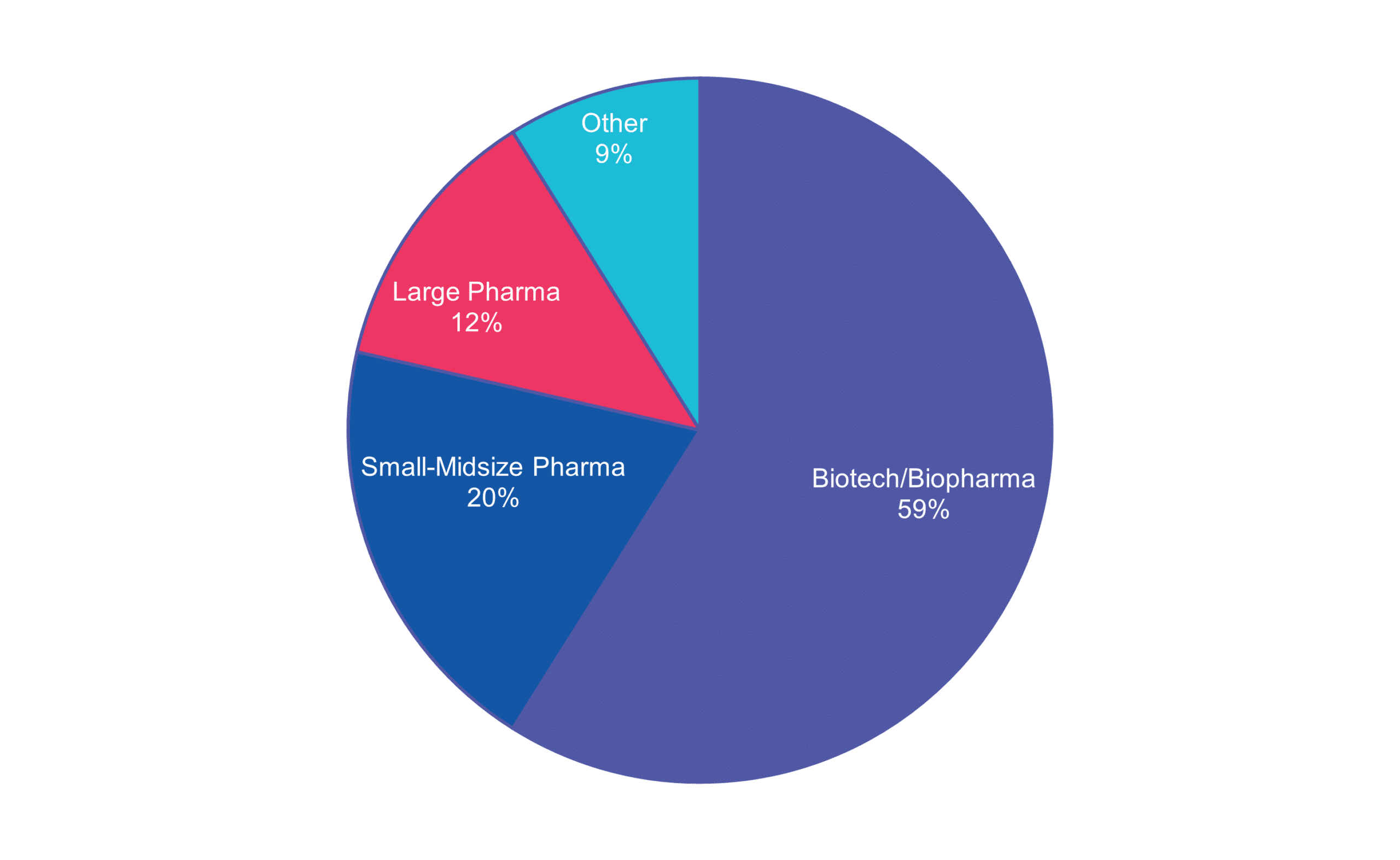
End-to-End Services Across Your Trial Continuum
Cerba Research has supported biopharma and pharmaceutical companies in developing metabolic therapies. As such, we collaborate mostly with biotech/biopharma companies

Our Areas Of Expertise In Metabolic Diseases
We have expertise in a plethora of metabolic indications, with NASH and type 2 diabetes being the most studied. We also participated in the approval and market expansion of 11 metabolic-related therapies marketed for various indications, such as, but not limited to, type 1 and 2 diabetes.
HbA1c Capabilities
Hemoglobin A1C (HbA1c) testing is a blood test that measures average blood glucose level over the course of 2-3 months (4). It is used to diagnose and monitor diabetic or prediabetic conditions and is an important study endpoint, reaching a surrogate level for the prevention of cardiovascular events, for diabetes and obesity trials. In other words, HbA1c should be considered while interpreting cardiovascular outcomes with new antidiabetic drugs (5). We offer HbA1c capabilities on a global scale for a high consistency of results for your global / regional trial. Did you know that Cerba Research Canada garnered the highest level of HbAc1 accreditation granted by the national glycohemoglobin standardization program (NGSP) HbAc1 level I certification? Reach out to learn more.
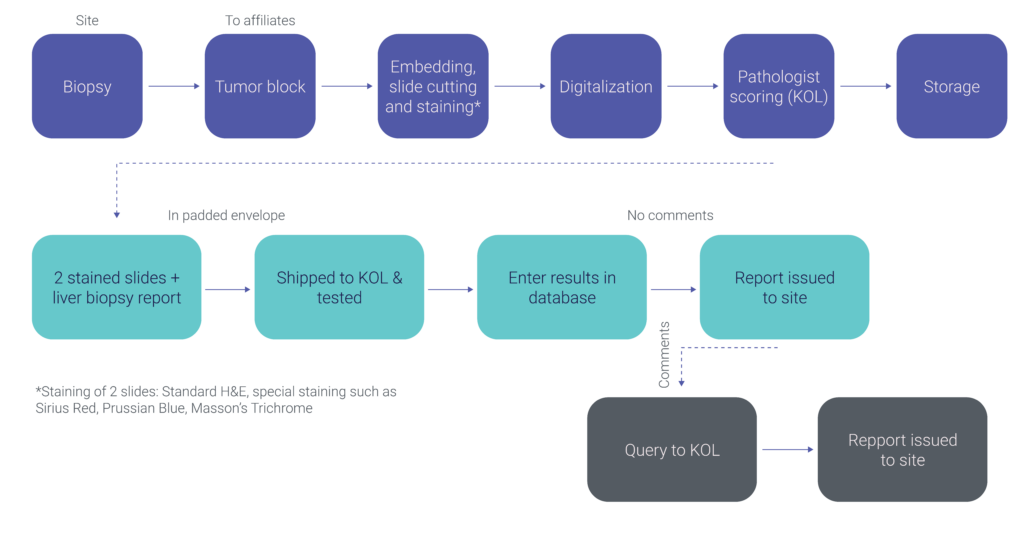
Liver Biopsy Evaluation In NASH Studies
Given our expertise in NASH and operational flexibility, we believe we are the ideal lab partner, making it our mission to support you in your fight against this chronic liver disease. Moreover, we combine our unique experience as a major international central laboratory with the cutting-edge technology of our partner, OWL metabolomics. Together, we provide a broad range of clinical support solutions for your NASH/NAFLD project. Check out our liver biopsy evaluation process for your NASH study.
Immunogenicity In Metabolic Trials
As with all biologics and biosimilars, there is a risk for an immune response in patients treated with this drug class. The immunogenicity data reflect the percentage of patients whose test results were considered positive for antibodies to biologics in immunoassays. As such, we offer long-standing scientific expertise in pharmacokinetics (PK) and immunogenicity (antibody-drug antibodies (ADAs) and neutralizing antibodies (NAbs)) with a standardized or a customized approach as per your vision.
Did you know that Cerba Research Canada was instrumental in immunogenicity validation (e.g. cell-based assay to measure the active fraction of the free drug and NAb assays) and clinical samples analysis that supported a vast area of clinical trials with a glucagon-like peptide 1 receptor agonist (GLP-1)? This GLP-1 agonist is currently marketed to improve glycemic control in patients with type 2 diabetes.
Our focus extends from peptides to mAbs, and more. In addition, we are good laboratory practice (GLP), college of American pathologists (CAP), clinical laboratory improvement amendments (CLIA) accredited for your regulatory requirements.
References
1. National Library of Medicine: Nutritional and metabolic diseases. URL [Nutritional and Metabolic Diseases – Genes and Disease – NCBI Bookshelf (nih.gov)].
2. National Institute of Health: Nonalcoholic Fatty Liver Disease (NAFLD) & NASH. URL [Nonalcoholic Fatty Liver Disease (NAFLD) & NASH – NIDDK (nih.gov)].
3. Selliah N, Nash V, Eck S, Green C, Oldaker T, Stewart J, Vitaliti A, Litwin V. Flow Cytometry Method Validation Protocols. Curr Protoc. 2023 Aug;3(8):e868. doi: 10.1002/cpz1.868. Erratum in: Curr Protoc. 2024 Jan;4(1):e988. PMID: 37606503.
4. CDC Diabetes Home: All about your A1C. URL [All About Your A1C (cdc.gov)].
5. Ambrosi P, Daumas A, Villani P, Giorgi R. Glycosylated Hemoglobin as a Surrogate for the Prevention of Cardiovascular Events in Cardiovascular Outcome Trials Comparing New Antidiabetic Drugs to Placebo. Cardiology. 2020;145(6):370-374. doi: 10.1159/000506004. Epub 2020 Feb 21. PMID: 32088710.
Discover Our Expertise In Transforming Research For Your Metabolic Therapies
We recommend starting engagement with our scientific team early, such as at the protocol design phase, for optimal results. Reach out to us here.