Cell Culture Solutions for IHC Development
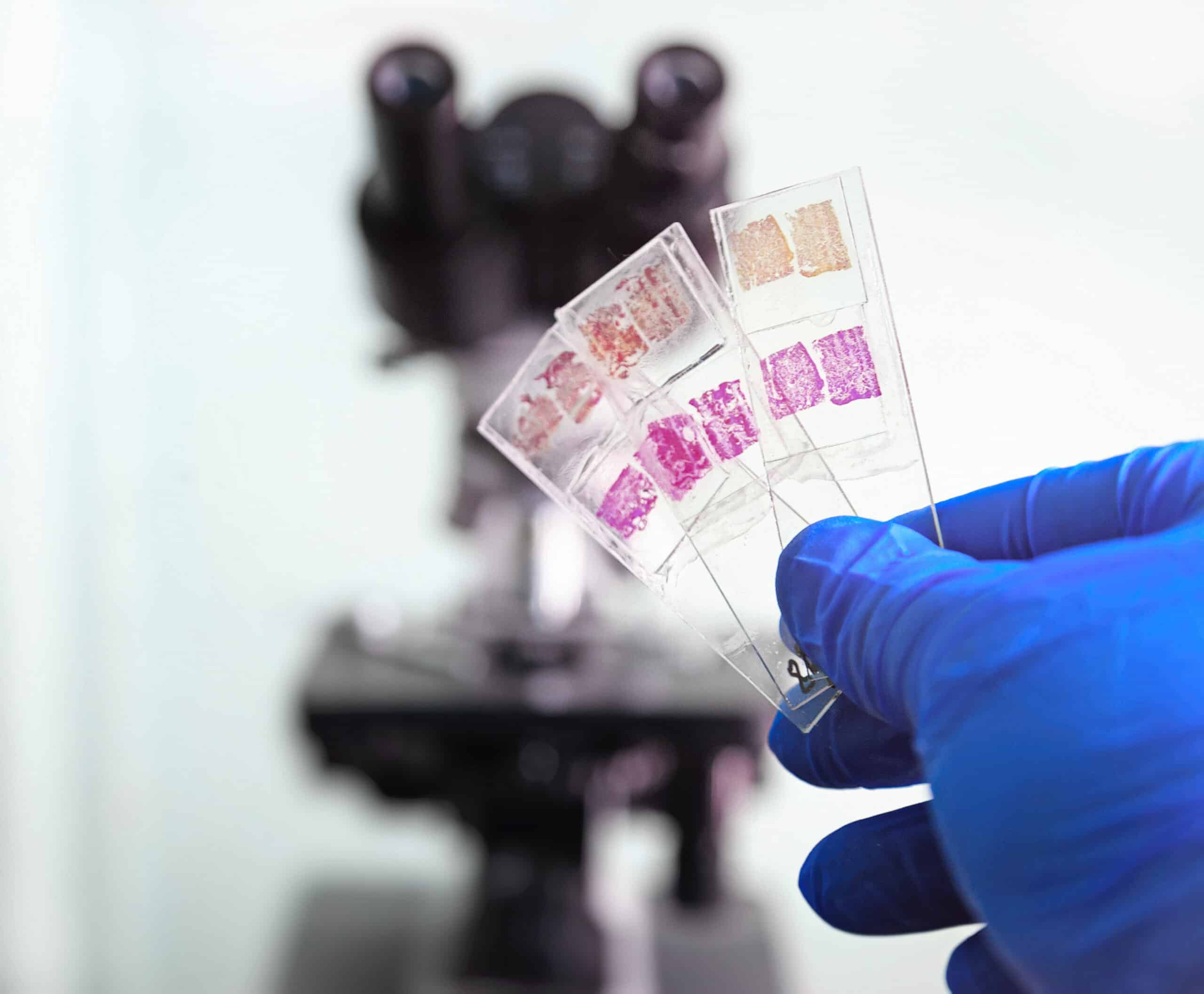
The development of innovative therapies often comes with the need for biomarkers that are not yet available as IVD-grade kits. Many research use-only (RUO) antibodies for IHC are made commercially available based on tissue/organ specificity only. Cerba Research recommends additional specificity testing of RUO antibodies before any program. Our histology-dedicated cell culture lab provides the best negative and positive controls for IHC specificity through transfection of a negative cell line with the gene encoding for each biomarker of interest.
Primary cell culture or culture of cancer cell lines for FFPE processing and IHC testing can also be provided.

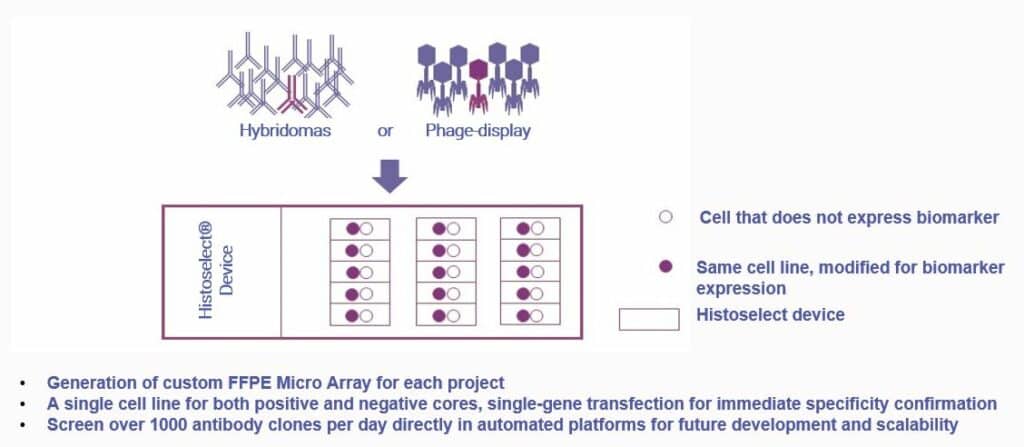
Histoselect®: The High-Throughput Solution for IHC Screening
Phage-display or hybridoma-based antibody generation of new antibodies can generate thousands of clones to be screened. With the limited sample number capabilities of automated IHC platforms and the time pressure to quickly identify leads among hundreds of growing hybridomas, a first screening step based on another method is often used in IHC antibody development programs.
There is, however, accumulated evidence that the best clones for ELISA or flow cytometry do not perform for IHC, and vice-versa, because of the antigenic modifications of the target proteins induced by formalin fixation in FFPE samples. Cerba Research has designed a proprietary solution for automated and high-throughput screening of large numbers of antibody clones in IHC format to select the best ones with the most relevant readout.
Discover How We Can Help You With Histoselect®
Reach out to our IHC antibody selection team and discover how we can help advance your research

