What Is Neuroscience?
Neurological diseases are conditions that affect the central (CNS) and peripheral nervous system (PNS). In other words, the brain, spinal cord, and nerves, which manage bodily, cognitive, behavioral, and emotional activities, may be impaired, causing a range of symptoms. There is a wide range of neurological disorders, such as dementias (e.g., Alzheimer’s disease), seizures (e.g., epilepsy), migraine, sleep disorders, anxiety and depressive disorders, muscle weakness, pain, and more. Such mental disorders affect 1 in 8 people worldwide (1).
Our research and development team aims to focus on the underlying mechanisms of neurological disorders as per your vision. As neurology development can be a demanding therapy area due to a distressed and stigmatized patient population (1), the highly complex human brain, and the high failure rate in late-stage trials (2), this requires a flexible and agile specialty laboratory partner who can adjust as the protocol does, ensuring safety and efficacy endpoints are captured appropriately and enabling studies to move forward with the right data at the right time. Cerba Research can provide a wide range of integrated specialty and safety testing to your existing central laboratory neuroscience processes. Contact us to find out.
Cerba Research Neuroscience Services
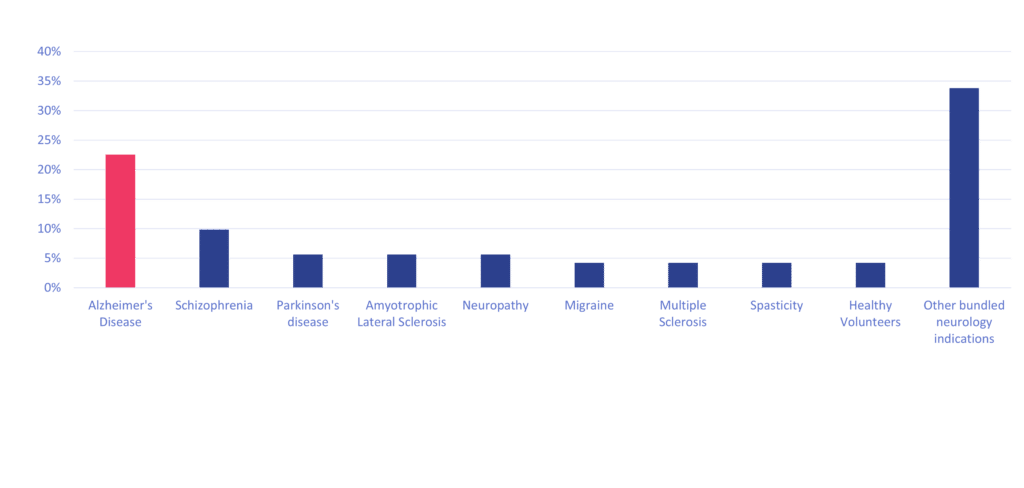
Cerba Research offers a wide range of expertise that can uniquely support neuroscience research and development as we offer end-to-end solutions from discovery to post-market authorization for your global / regional trials. In the past 10+ years, Cerba Research conducted 70+ neuroscience trials, most of which included phase II and III studies. Notably, the lab has played a pivotal role in the market authorization or expansion of 15 innovative drugs indicated for Alzheimer’s disease, schizophrenia, major depressive disorders, and spinal cord injuries, amongst other indications.
Our team is experienced in many innovative techniques. We have a global network of laboratories performing routine tests every day for medical diagnostics and we are offering a wide range of capabilities, ranging from safety testing (also known as routine testing) to globally harmonized ligand-binding assays (LBAs), flow cytometry (FCM), spatial omics, immunogenicity, genetics / genomics (next-generation sequencing (NGS)), and more. We also facilitate state-of-the-art technologies, subject matter expertise, specialized logistics, and operations to accelerate your neuroscience research and development.
Thanks to our expertise in assay development, validation (3), customization capabilities, robust kit building, sample management, and logistics, we have garnered about 21,700+ patients screened and 16,000+ randomized from our neuroscience programs since 2012.
We Perform Specialty Testing In ~45% Of Our Neurosciences Portfolio
We have had 70+ neuroscience trials since 2012 and are growing. We perform specialty testing in ~45% of cases within those trials. Specifically, we can perform a plethora of enzyme-linked immunosorbent (ELISA) assays, enzyme immunoassays (EIA), electrochemiluminescence immunoassays (ECLIA), ELLA and mesoscale discovery (MSD) assays on serum, plasma and/or cerebral spinal fluid (CSF). We can also perform intracellular cytokine detection by flow cytometry (FCM) and own a large offering of next-generation sequencing (NGS) neurology comprehensive panels (e.g., up to 2141 genes involved in neurological disorders), amongst other innovative techniques. We can also perform any routine testing in-house for safety surveillance, such as, but not limited to, coagulation, biochemistry, urinalysis, pregnancy test, COVID testing, and serology, which are essential for any neuroscience trials and patient inclusion / exclusion criteria.
Learn More About Our Neurosciences Drug Development Capabilities
NGS, broad panels, custom panels
RNA seq
Single-gene
ddPCR, qPCR
Whole exome / whole genome
NanoString®
SNP-array
DNA / RNA extraction
Streck Cell-Free DNA BCT®
PaxGene®, Qiamp kits
Coagulation
Hematology
Biochemistry
Urinalysis
Pregnancy test
COVID test
Serology
Thyroid function
HbA1c
sPEP, uPEP
Multiplex cytokine profiling (37-plex)
50+ ligand binding assays
AB42, TAU and pTAU proteins in CSF
ELISA
ELLA
ECLIA
MSD
PK / ADA / Nab
FCM
Cytek Aurora
Immunophenotyping (including intra-cell markers)
Receptor occupancy
Intracellular cytokine detection
Optical genome mapping, our next-generation cytogenetics
PK / ADA / Nab
Multiplex / simplex IHC
250+ biomarkers / protocols
Full histopathology service
Halo®, Visiopharm®
Board certified pathologists
Large biobank
NanoString® GeoMx, FISH, ISH
End-to-End Services Across Your Trial Continuum
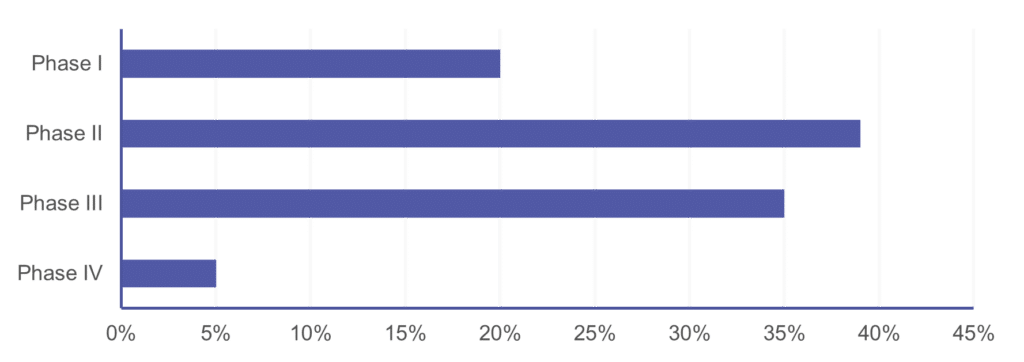
Cerba Research can execute upon every neuroscience trial phase, ranging from discovery / pre-clinical (data not shown) to post-market authorization trials. As such, our most decisive experience is with phase II and III trials, which comprise nearly 75% of our neurology portfolio. Cerba Research is also active early in neuroscience-related trials, where our sponsors often continue to work with us on full-asset programs until registration trials and beyond. This intelligence, along with custom assays and on-target protocol advice, can accelerate your program to market.
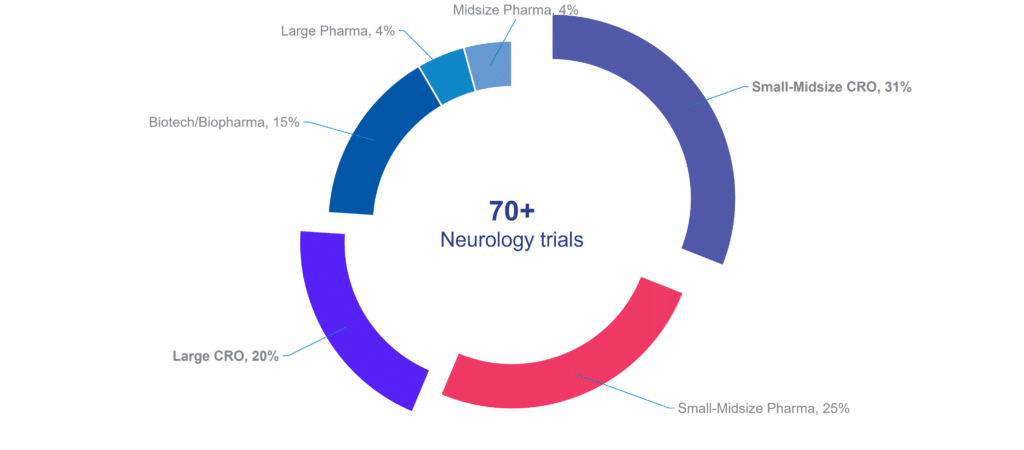
Cerba Research has supported not only biopharma and pharmaceutical companies but also other contract research organizations (CROs) in developing neurology therapies. Our heritage in specialty labs and our experience in central lab services enable us to develop research techniques to underpin the next generation of clinical trials. A new kind of research where diagnostics are driven by clinical data and insights, supported by specific therapeutic expertise.
Approval Of An Innovative Alzheimer’s Disease mAb
Cerba Research participated in registration trials where we performed important CSF assays for a mAb that garnered a recent regulatory market authorization. As such, Cerba measured “the core CSF biomarkers of neuronal degeneration including Beta-amyloid 42 protein (Aβ42), total tau protein (t-tau) and phosphorylated tau protein (p-tau)” (4) with a specific ELISA kit. CSF samples were garnered from nearly 600 patients to properly diagnose and stage the disease.
Genetics & Genomics For Neuroscience Indications
At Cerba Research, our expert team can optimize neuroscience trials due to our extensive experience in genomics and access to a full range of sophisticated instruments. Benefit from a large offering across different neurological disorders with our Cerba NGS comprehensive panels. It covers mutations with established, emerging & exploratory value across Parkinson’s, epilepsy, intellectual disabilities, migraines, amyotrophic lateral sclerosis (ALS), and more. Our genetics and genomics workflow from sample collection to reporting takes 5-15 business days, depending on panel complexity and geographic location.
Major Depressive Disorders Test That Combines Artificial Intelligence & Real-world Evidence
PREDICTIX® is designed to select the most suitable antidepressant treatment of choice for patients suffering from major depressive disorders (MDD). Based on the patient’s combined genetics and socio-demographic characteristics, PREDICTIX® may predict the probability of response and possible side effects encountered with MDD medicines.
Adeno-associated Virus 9 (AAV9) Testing
Gene therapies may address the root cause of a genetic disorder by either restoring the function of a missing gene or by blocking the function of a detrimental mutant gene. Gene therapy is a useful approach to treat diseases caused by a single gene defect (5). Modified viruses (aka vectors) are a suitable way so that the therapeutic genetic material can be transported to the diseased cells (6). As such, many different assays are required to measure safety and efficacy of such gene therapies. One of them is referred to as AAV9 testing, where the Cerba anti-AAV9 human IgG ELISA is a tool used to determine the presence of antibodies against AAV9 in serum.
The anti-AAV9 human IgG ELISA-based test is intended for an in vitro semi-quantitative estimation of anti-AAV9 IgG antibodies titers directed against AAV9 capsid particles that are present in the patient serum sample. This assay is intended for assessing the presence of anti-AAV9 antibodies in patients (including newborns and infants) diagnosed with spinal muscular atrophy (SMA). Antibody titer levels are used to make an appropriate treatment decision, as high titers are prohibited from administering the novel gene therapy. As SMA is a rare, devastating neuromuscular disease that requires a fast turnaround from sampling to test results, we can report on anti-AAV9 within 2 business days on average.
Immunogenicity In Neuroscience
With our in-depth biologics and biosimilar experience, we support our clients from pre-clinical to post-market authorization. We offer long-standing scientific expertise in pharmacokinetics (PK) and immunogenicity (antibody-drug antibodies (ADAs) and neutralizing antibodies (NAbs)) with a standardized or customized approach, as per your vision. Our focus extends from mAbs to antibody-drug conjugates and more. In addition, we are good laboratory practice (GLP), college of American pathologists (CAP), clinical laboratory improvement amendments (CLIA) accredited for your regulatory requirements.
References
1. World Health Organization: Mental disorders. URL [https://www.who.int/news-room/fact-sheets/detail/mental-disorders].
2. Hyman SE. Revolution stalled. Sci Transl Med. 2012 Oct 10;4(155):155cm11. doi: 10.1126/scitranslmed.3003142. PMID: 23052291.
3. Selliah N, Nash V, Eck S, Green C, Oldaker T, Stewart J, Vitaliti A, Litwin V. Flow Cytometry Method Validation Protocols. Curr Protoc. 2023 Aug;3(8):e868. doi: 10.1002/cpz1.868. Erratum in: Curr Protoc. 2024 Jan;4(1):e988. PMID: 37606503.
4. Non-invasive novel biomarkers to predict Alzheimer’s risk. URL [https://cerbaresearch.com/publications/non-invasive-novel-biomarkers-to-predict-alzheimers-risk].
5. Gonçalves GAR, Paiva RMA. Gene therapy: advances, challenges, and perspectives. Einstein (Sao Paulo). 2017 Jul-Sep;15(3):369-375. doi: 10.1590/S1679-45082017RB4024. PMID: 29091160; PMCID: PMC5823056.
6. DiMattia MA, Nam HJ, Van Vliet K, Mitchell M, Bennett A, Gurda BL, McKenna R, Olson NH, Sinkovits RS, Potter M, Byrne BJ, Aslanidi G, Zolotukhin S, Muzyczka N, Baker TS, Agbandje-McKenna M. Structural insight into the unique properties of adeno-associated virus serotype 9. J Virol. 2012 Jun;86(12):6947-58. doi: 10.1128/JVI.07232-11. Epub 2012 Apr 11. PMID: 22496238; PMCID: PMC3393551.
Discover Our Expertise In Transforming Research For Your Neuroscience Therapies
We recommend starting engagement with our scientific team early, such as at the protocol design phase, for optimal results. Reach out to us here.