Cerba Research provides the solutions to challenges in laboratory testing for Cell & Gene Therapy
The fast-paced Cell & Gene Therapy landscape brings the potential for health improvements for patients waiting for appropriate personalized treatments. As such, Cell & Gene Therapy biotech and pharma communities face the challenge of bringing novel therapeutics to the market within tight deadlines. Meeting the demands of Cell & Gene Therapies across all stages of the drug development process requires a strategic partner capable of dealing with highly complex clinical trial challenges.
As a specialty laboratory solutions provider, Cerba Research is at the forefront of addressing the complexity in Cell and Gene Therapy clinical trials and offers a comprehensive suite of solutions.

Project management expertise with Cell & Gene Therapy specific experience
Cerba Research provides end-to-end project management from sample receipt to data reporting, emphasizing customized assay development and logistics. Our scalable solutions ensure consistency, supported by proactive risk and contingency management for Cell & Gene Therapy clinical trials.
Access to a scientific and specialist network
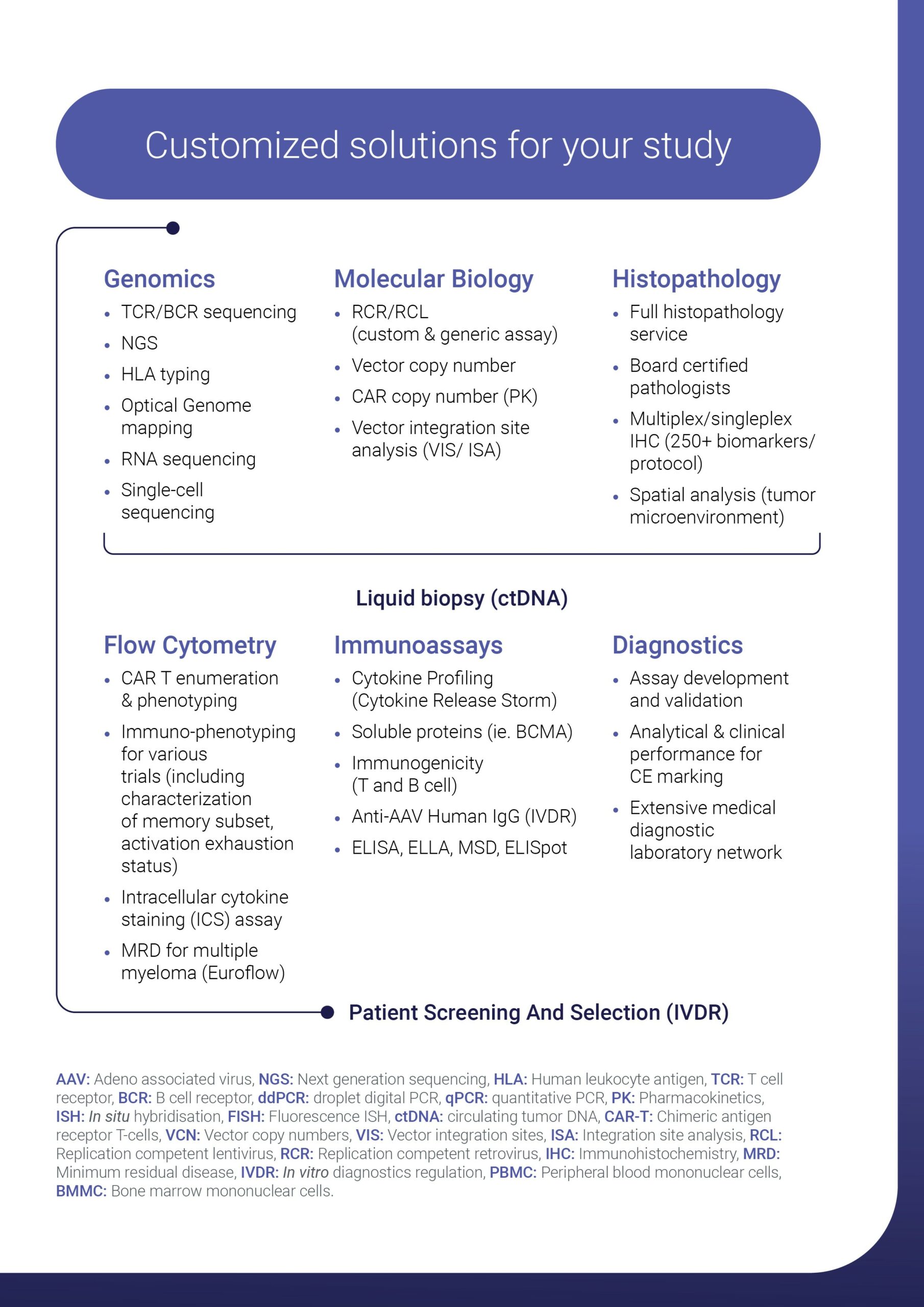
Cerba Research offers a comprehensive scientific network. We specialize in genomics, molecular biology, flow cytometry, immunology, histopathology, virology, and diagnostics. In addition, we provide logistics account management to streamline sample and kit distribution, ensuring efficient clinical trial operations.
Extensive assay offering and testing customization
Our extensive assay offering aligned to relevant regulatory guidance, such as In Vitro Diagnostic Regulation (IVDR), includes a wide range of research areas, such as rare diseases, oncology, autoimmune diseases, neurodegenerative disorders, and metabolic conditions.
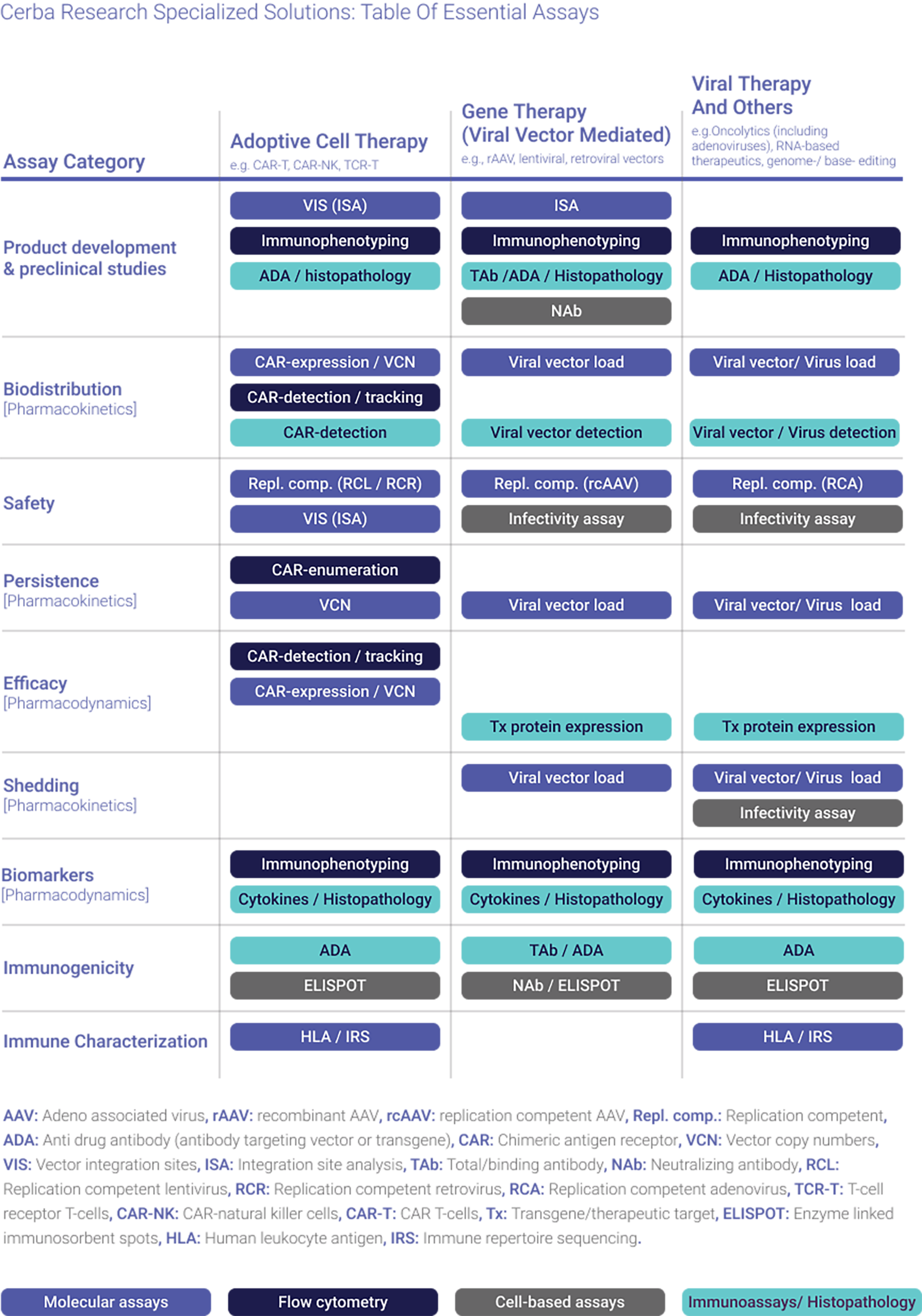
Global specialty laboratory with central laboratory network
Cerba Research provides access to specialized testing for Cell & Gene Therapy trials, supported by global central laboratory services. Our in-house molecular biology, genetics, biomarkers, histopathology, and flow cytometry capabilities are available in North America, Europe, Asia-Pacific, and Africa. In addition, we offer a global network comprising:
- Pre-processing labs
- PBMC/ BMMC isolation network
- Research, and partner laboratories
Customized logistics solutions for sample and kit management
Cerba Research provides tailor-made logistics solutions for managing samples and kits in Cell & Gene Therapy trials. Our multiple service levels include standard and premium, while a 24/7 helpdesk offers continuous support for contingency planning and seamless sample handling. We have the agility to adapt to both regional and global regulations (IATA/ADR compliant).
Learn more about our logistics capabilities here.
AAV immunogenicity screening
- Various assays and platforms are available, including ELISA, MSD platform, NAb, and more.
- In-house produced and quality controlled IVD and IVDR compliant sample and assay kits.
- Dedicated account management team for 24/7 logistics support and diagnostic services.
- Rapid turn-around times (average 1-5 days) from sample pick up to reporting.
- A network of satellite analytical labs for AAV screening in remote regions.
Learn more here and download our AAV case study, a multi-centre prospective and validation study.
Prioritizing Safety in CAR-T Therapy: Patient Monitoring with Cerba Research’s Testing Portfolio
In this white paper, we highlight several of our
capabilities in this field, including:
• Vector copy number (VCN) testing by droplet
digital PCR (ddPCR)
• Viral integration site (VIS) analysis by
sequencing
• Replication competent virus testing by
quantitative PCR (qPCR)
• Enumeration of CAR+ T cells in blood
circulation and immunophenotyping of CAR+
T cells and CAR- T cells by flow cytometry
(FCM)

Cell & Gene Therapy Laboratory Solutions
Cerba Research provides access to a
network of specialized testing laboratories
for cell and gene therapy trials. Our
dedicated project teams, supported by a
global footprint and customizable logistics
capabilities, ensure responsiveness and
agility. Additionally, our in-house scientific
solutions include genomics, molecular biology, flow cytometry , immunology, histopathology, virology and diagnostics.
Discover Our Expertise In Transforming Research For Your Next Generation CGT
Relevant Content
Reach out to our experts here.