What Are Rare Diseases?
There is no single, widely accepted definition for rare disorders. For instance, according to the World Health Organization (WHO), “the international classification disease-11 (ICD-11) includes some 5,500 rare diseases and their synonyms” (1). According to the U.S. Food & Drug Association (FDA), “over 7,000 rare diseases affect more than 30 million people in the United States” (2). In addition, there are also regional differences. For instance, in the U.S., rare diseases are defined as “a disease or condition that impacts fewer than 200,000 people” (3). In Europe, it’s “as those with a prevalence lower than 5 in 10,000 people” (4-5) and in Japan, it’s “as those with a prevalence of less than 50,000, or one in 2,500” (6).
Developing rare disease therapies requires increased access to expertise, knowledge, data, and insights to bring the right patient to the proper treatment and/or trial at the right time. We facilitate state-of-the-art technologies, subject matter expertise, specialized logistics, and operations to accelerate your rare disease research and development.
Rare Diseases Are A Priority In The Clinical Trials Landscape
Rare disease research and development is a priority for drug developers, patients, regulators, non-profit organizations, and healthcare providers. However, our sponsors face significant challenges when trying to advance rare disease research and innovative treatments because of the following:
- A lack of consensus on endpoints.
- Difficult to diagnose diseases.
- Difficult to identify patients.
- Lack of gathered scientific knowledge and information.
- Inexperienced, understaffed, or unavailable research centers.
- A geographically dispersed patient population.
We Perform Specialty Testing In About 80% Of Cases Within Those Trials
Specifically, we can perform the below state-of-the art technologies for your rare disease trial
Validated assays to detect antibodies against adeno-associated vector (AAV) and target inserts using antibody-ELISA assays. Immune response by monitoring cytokines via mesoscale discovery (MSD) platform and cytokine release syndrome (CRS) panel.
BD FACS Canto & Lyric, Cytek Aurora for minimal residual disease (MRD) detection, enumeration, immunophenotyping, cell sorting & isolation, tumor microenvironment, intracellular cytokine detection.
Peripheral blood mononuclear cells (PBMC), bone marrow mononuclear cell (BMMC), CD138+…..PBMC processing in 25+ countries with 45+ processing labs and growing.
Next-generation sequencing (NGS) with formalin-fixed paraffin-embedded (FFPE) and liquid biopsies (e.g. circulating tumor DNA (ctDNA)). Custom panels and already existing broad-panel assays for rare cancers/rare disorders.
Detecting and quantifying genes/viral genome expression (replication competent lentivirus (RCL) and vector copy number (VCN)).
Fluorescent in situ hybridization (FISH), NanoString® & immunohistochemistry (IHC) with 250+ IHC protocols available for analysis.
Cerba Research has also the ability to support drug development from pre-clinical to post-market authorization with immunogenicity assays for your biologics, biosimilars & cell and gene therapies (CGTs).
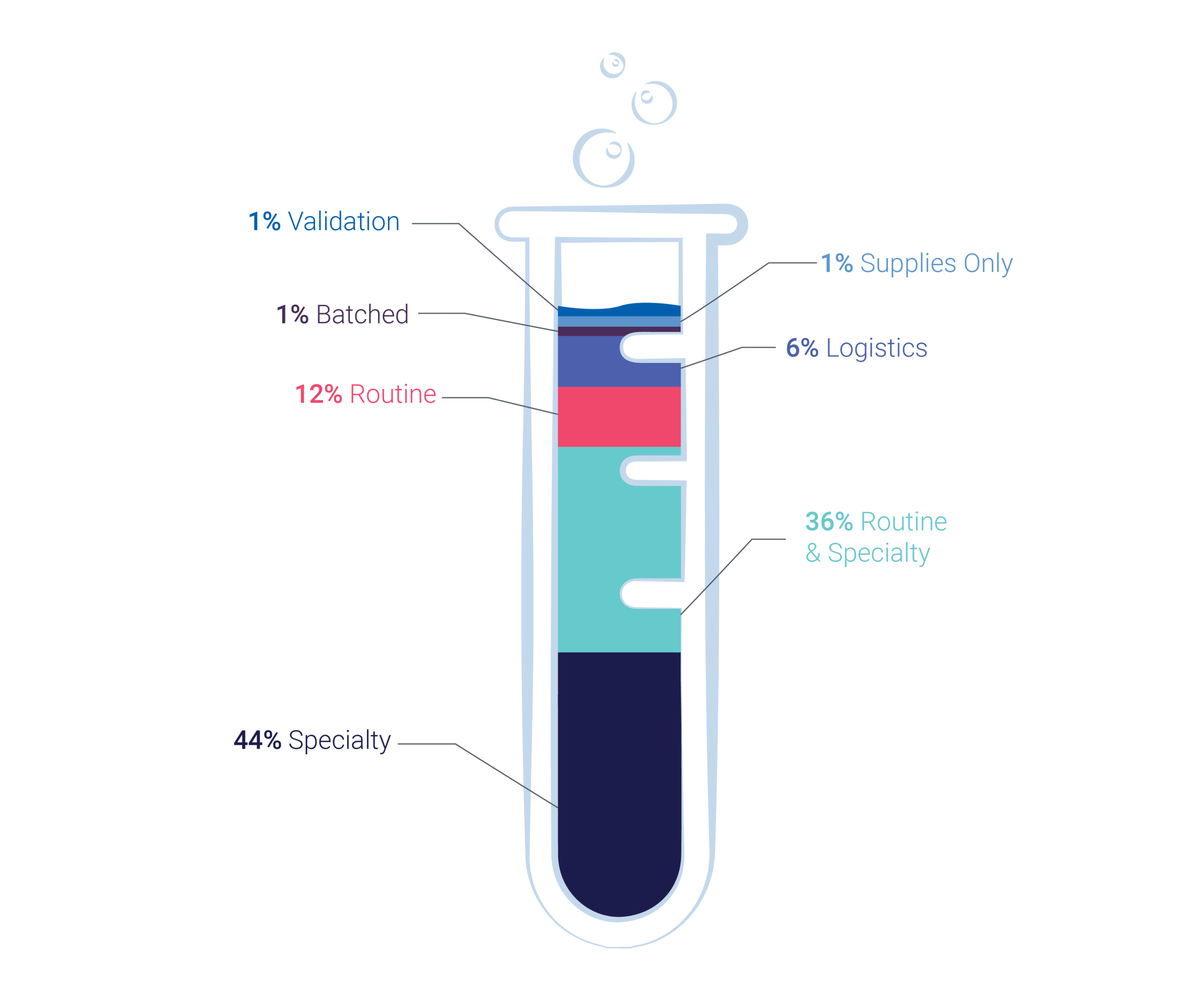
We can also perform any routine testing (aka safety testing) centrally which are essential for any rare disorder trial, such as, but not limited to:
- Coagulation
- Biochemistry
- Urinalysis
- Pregnancy test
- Serology
- And more
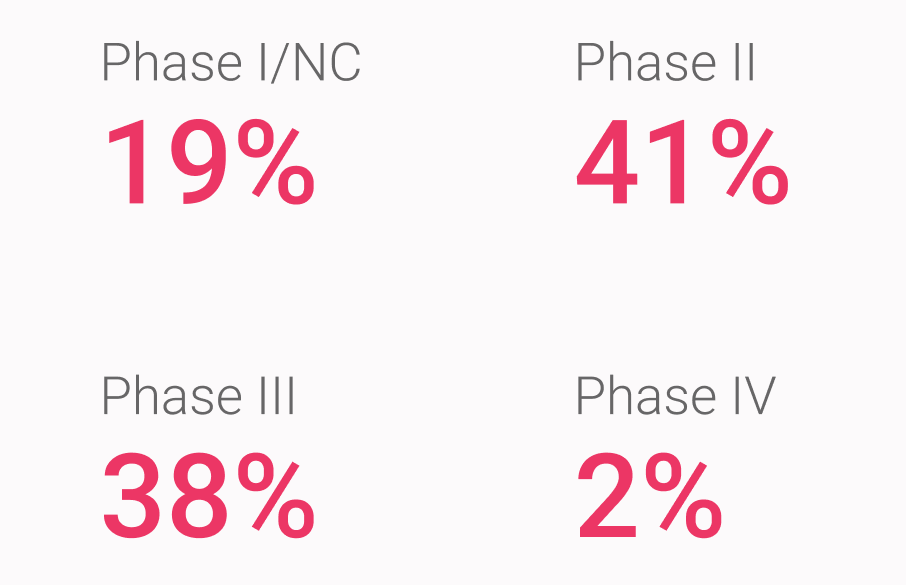
Cerba Research can execute upon every rare disease trial phase, ranging from discovery/pre-clinical (data not shown) to post-market authorization trials. As such, our most decisive experience in rare disorders is with registration trials (phase III) and phase II trials, which comprise close to 80% of our rare disease trial portfolio.
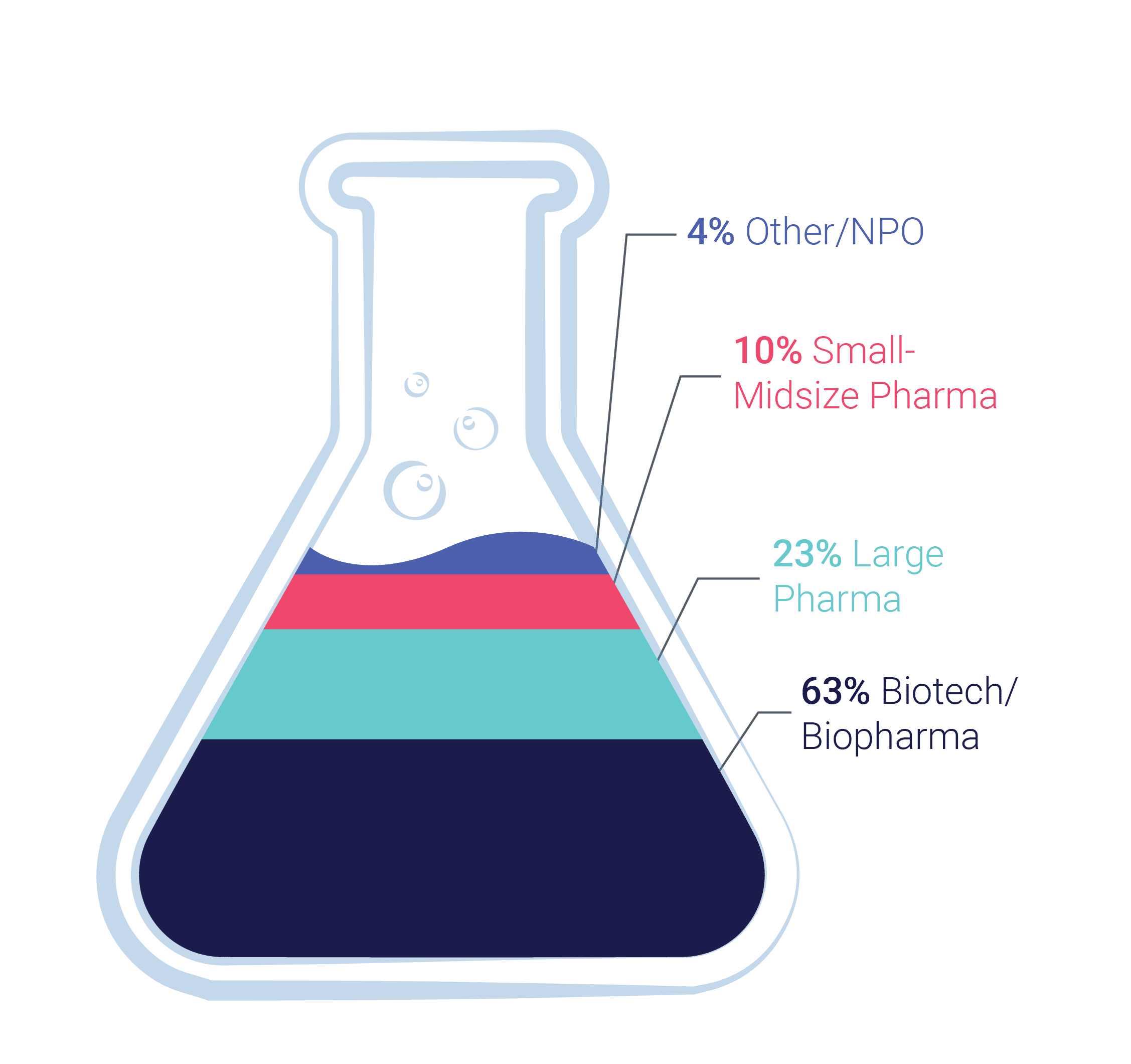
Cerba Research has supported biopharma and pharmaceutical companies in developing rare disease therapies (for more than 30 years). Our heritage in specialty labs and our experience in central lab services enable us to develop research techniques to underpin the next generation of clinical trials. A new kind of research where diagnostics are driven by clinical data and insights, supported by specific therapeutic expertise.
Our Areas Of Expertise In Rare Diseases
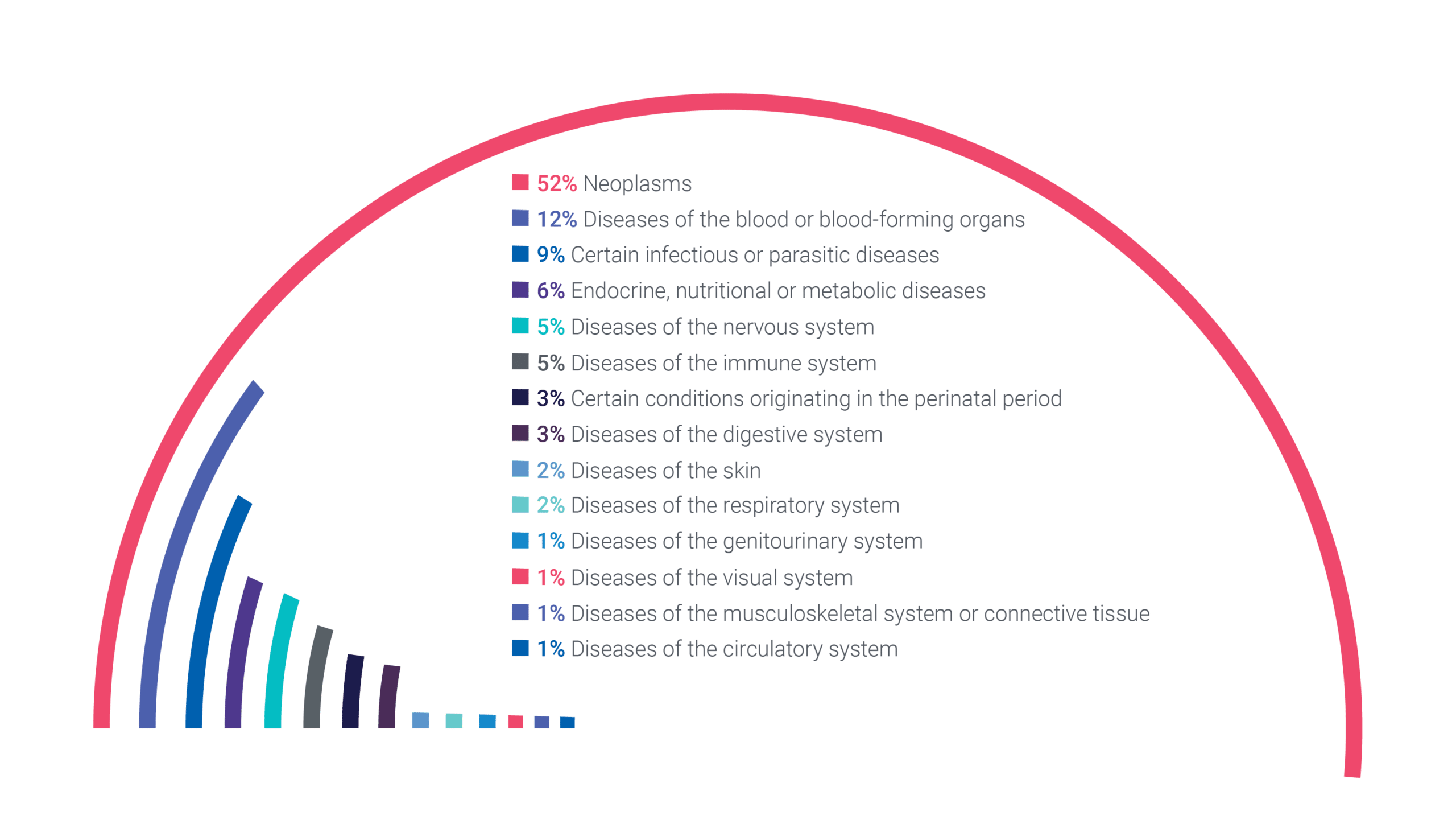
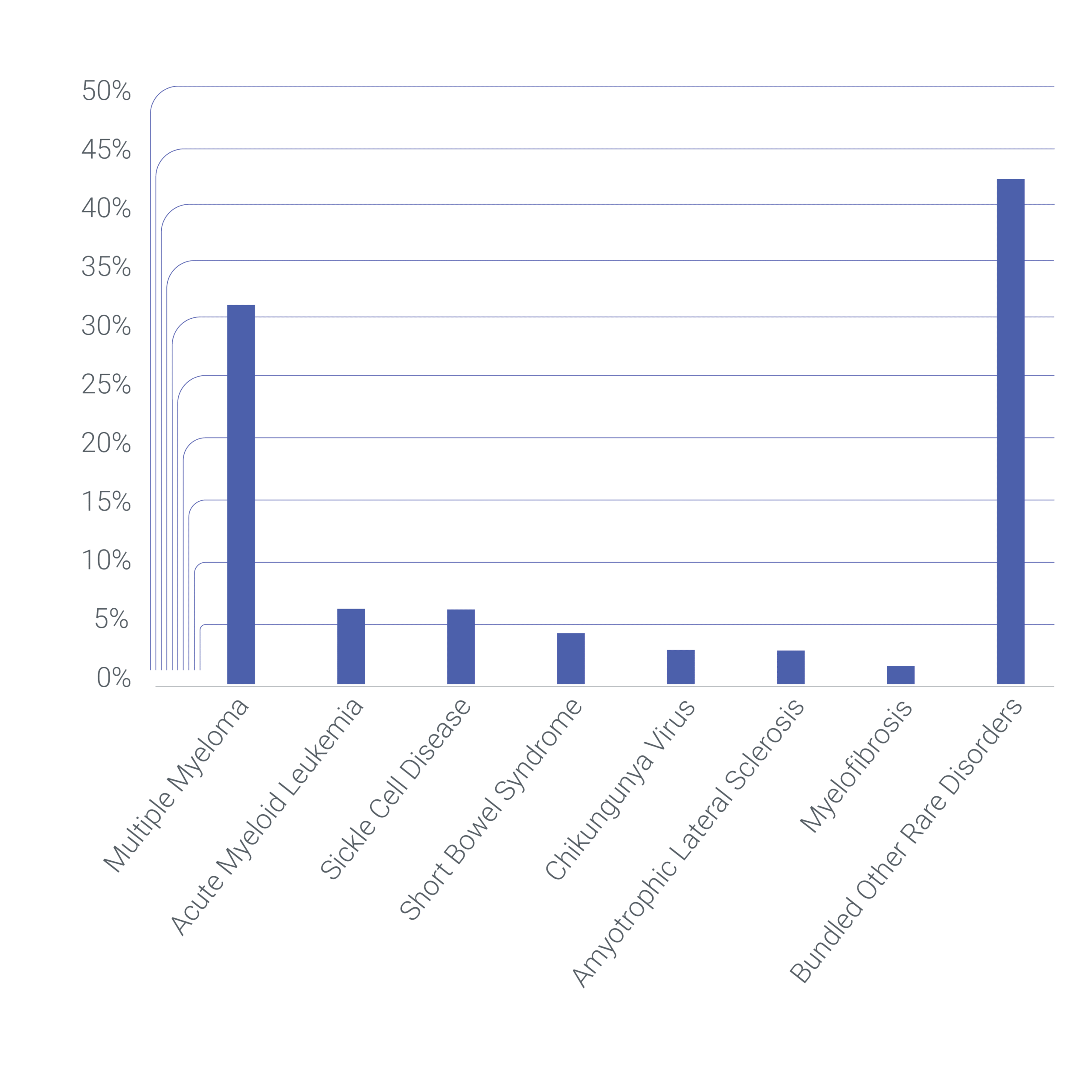
Based on the orphanet (7) and the ICD 11 classification (1), Cerba Research has the most robust rare disease therapy experience in oncology, with 52% of our research focusing on rare neoplasms. Relapse and/or refractory multiple myeloma is the most common indication within our portfolio of rare disorder trials. However, our experience extends to various other ICD11 classification areas, such as:
- Diseases of the blood or blood-forming organs.
- Diseases of the nervous system.
- Endocrine, nutritional or metabolic diseases.
- Certain infectious or parasitic diseases.
Cerba Research Rare Disorder Trials By ICD 11 Classification
Cerba Research Rare Disorders Trials By Indication
References
1. World Health Organization: Rare diseases. URL [https://www.who.int/standards/classifications/frequently-asked-questions/rare-diseases].
2. U.S. Food & Drug Association: Medical products for rare diseases and conditions. URL [https://www.fda.gov/industry/medical-products-rare-diseases-and-conditions].
3. National Center for Advancing Translational Sciences: Genetic and rare diseases information center. URL [https://rarediseases.info.nih.gov/about].
4. Rath A, Olry A, Dhombres F, Brandt MM, Urbero B, Ayme S. Representation of rare diseases in health information systems: the Orphanet approach to serve a wide range of end users. Hum Mutat. 2012 May;33(5):803-8. doi: 10.1002/humu.22078. Epub 2012 Apr 6. PMID: 22422702.
5. European Medicines Agency: Orphan medicine. URL [Orphan medicine | European Medicines Agency (europa.eu)].
6. Hayashi S, Umeda T. 35 years of Japanese policy on rare diseases. Lancet. 2008 Sep 13;372(9642):889-90. doi: 10.1016/S0140-6736(08)61393-8. PMID: 18790305.
7. Orphanet: The portal for rare diseases and orphan drugs. URL [https://www.orpha.net].
Download One Of Our Materials To Learn More About Our Solutions For Rare Diseases
Reach out to our experts to see how we can help you advance your research and development in rare diseases



