Our PBMC Processing Services
Joined together, our capabilities and expertise guarantee customers a high level of quality from sample collection to processing, shipping, handling, storage and analysis worldwide.
The usefulness and efficiency of PBMC studies should not be overlooked. The increased use of these immune mononuclear cells as a promising indicator of drug efficacy in clinical trials, as well as the increased convenience of working with samples versus conducting testing, makes PBMCs crucial. At Cerba Research, we embrace the potential of PBMC activities to maximize impact in clinical research.
To keep up with rising clinical research demand, we perform various PBMC activities like isolation from whole blood samples and DNA extraction using advanced technologies and equipment.
- PBMC isolation on a global scale
- Harmonized SOP/QC and instrumentation
- Utilize density grade centrifugation (DCG) with CPT® tubes
- For use in immunology, infectious disease, hematological malignancies, vaccine development, transplant therapy, personalized medicine, cell & gene therapy and toxicology.

Brochure – What Are The Main Challenges And Questions To Overcome With PBMCs?
There are important questions you need to ask yourself and any potential laboratory partner before you start a study including PBMC assays as part of your protocol :
- What PBMC methodology best fits your intended outcome and study?
- Where do you want to run your study and at what scale?
- What data are you looking to generate from your PBMC?
Your partner PBMC laboratory needs to be able to support you with their answers to all these challenges. Download our free brochure and discover how Cerba Research can help you answer these questions and help provide you with a global solution for all your PBMC needs.
- Management of sample processing labs
- PBMC personal training program and certification
- Custom sampling kits preparation
- Sample tracking and tracing (e.g. guaranteeing a temperature-controlled supply chain and online-sample timeline)
- Premium courier transportation in accordance with ADR & IATA regulations
- All our pre-processing labs are accredited to the relevant local laboratory accreditation standards, which include: CAP, GCP, GLP, ISO17025/15189
- Quality control and personnel training is monitored on-site to ensure compliance with the processing procedures required by the customer
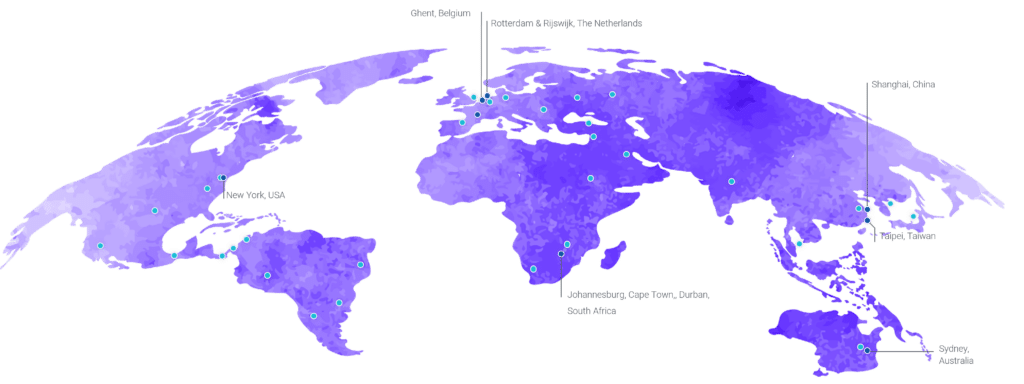
- 45+ processing labs in America, Europe, Africa, Asia, and Australia
- Ability to on-board lab partners worldwide
- PBMC isolation in 25+ countries
- Our standard PBMC processing protocol includes Vacutainer® CPT Mononuclear Cell Preparation Tube
- Customized upon request
- Short turnaround times
- Same-day PBMC isolation
Turnaround times may vary from one country to another, reflecting possible transportation delays or excessive workloads within the lab
Discover How We Process PBMCs

Reach out to our experts and discover how we can help you transform research and advance health
Contact Us
