Your Vision, Our Focus
Cerba Research’s clients are supported from pre-clinical to post-market authorization thanks to our in-depth biologics and biosimilars experience. We offer long-standing scientific expertise in pharmacokinetics (PK) and immunogenicity (antibody-drug antibodies (ADAs) and neutralizing antibodies (NAbs)) with a standardized or a customized approach to reflect your vision. Our focus extends from cell and gene therapy, to engineered proteins (including glycoPEGylated proteins (PEG)), monoclonal antibodies (mAb), antibody-drug conjugates (ADCs) and virus-like drug conjugates (VDC). In addition, we are compliant with good laboratory practice (GLP), college of American pathologists (CAP), and clinical laboratory improvement amendments (CLIA), among others for your regulatory requirements.
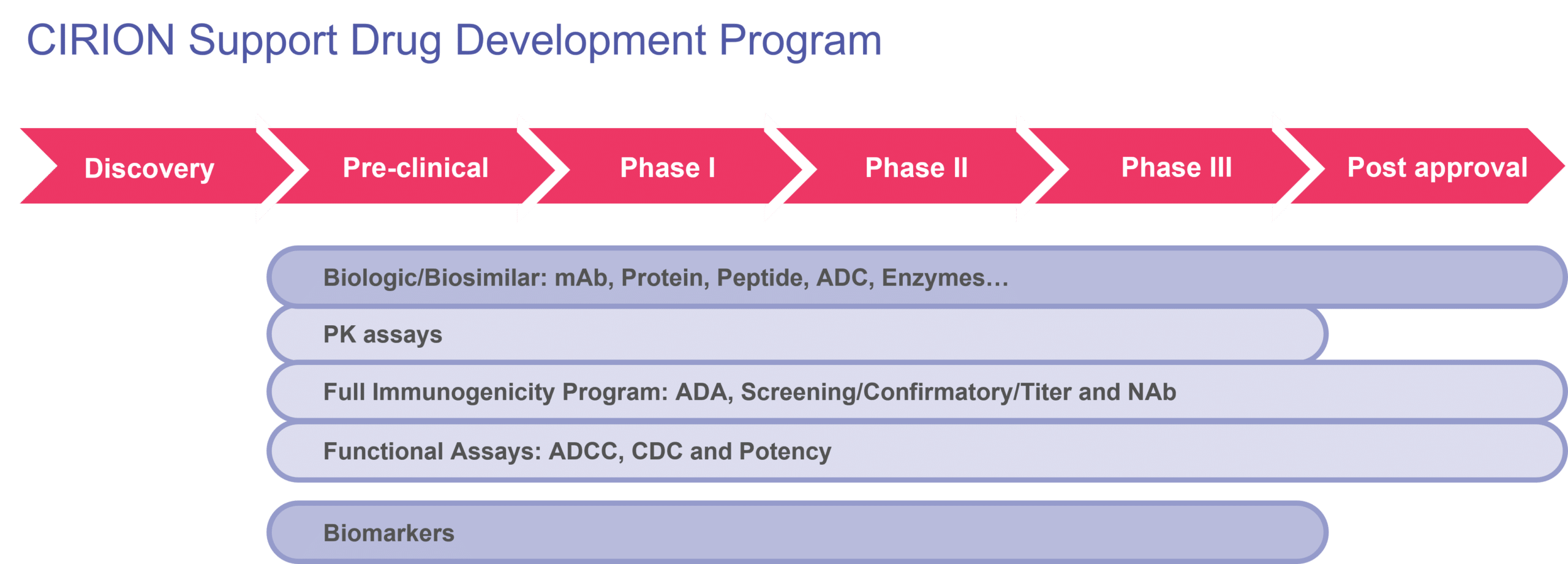
Dedicated Scientific Staff From Start To Finish
Our knowledge is extensive when it comes to PK and immunogenicity solutions for biologics and biosimilars. We have more than 25 years of recognized expertise in multinational complex GLP/GCLP projects. As a result, your project is in safe hands with our project managers, principal investigators, and experienced scientists. Each of them has various areas of expertise, including, but not limited to, immunology, virology, oncology, neurology, biology, molecular biology, and biochemistry.
All our laboratory staff hold a university degree and 60% have postgraduate degrees (M.Sc., Ph.D.). You can count on the same team from the kick-off of your immunogenicity project up to the finish line, which is one of our key differentiators. This ensures no loss of acquired competence throughout your project(s).
Our scientists are passionate and their long-standing aptitude in troubleshooting offers flexible and agile solutions to your R&D projects. Need to talk scientist to scientist? We work with you collaboratively and our team is always ready to discuss ideas, share their expertise and insights, and give you the peer-to-peer support you need. Reach out to us here.

Our Drug Class Experience
Cerba Research has experience in method transfer, development, and validation of PK and immunogenicity (ADA and NAb) methods for biologics and biosimilars across therapeutic areas. Our expertise expands from mAbs, pegylated agents, ADC, bi-specific antibodies, enzymes, peptides, vaccines, and cell and gene therapies. Assays are designed according to your asset’s structure, its function(s), and specific target(s) (e.g. mechanism of action).
Your Partner Of Choice
• You will have direct access to our scientists. We believe that the most important aspects of a successful collaboration are responsiveness, transparency and trusting communications.
• Our scientists have experience with mAbs, engineered proteins, peptides, ADCs, enzymes, toxins, and more for 25+ years.
• Cerba Research has developed and validated PK/ADA/NAbs assays for many biologics and biosimilars.
• Our team contributed to the market authorization of numerous biologics and biosimilars approved for many indications such as, but not limited to, breast cancer, neutropenia, anemia, and multiple sclerosis.
• We apply a fit-for purpose approach in providing method development, partial or full validation in a GxP, CAP, and CLIA-compliant manner as per your bioanalytical needs.
• We manage all aspects of sample analysis, reception, management, processing, and storage in a controlled environment aligned with your regulatory requirements.
• For instance, Cerba Research performed the development of PK and immunogenicity assays, from pre-clinical to post-market approval, of a filgrastim biosimilar, which is now approved worldwide.
Cerba Research Biologics And Biosimilars Services

Pharmacokinetics (PK)
- PK, sometimes described as what the body does to a drug, refers to the movement of drug into, through, and out of the body.
- Also referred to as the time course of its absorption, bioavailability, distribution, metabolism, and excretion.
- We routinely measure PK for biologics and biosimilars, with specific expertise in mAb, peptides, ADC, multivalent polymer conjugates, VDC, growth factors and glycoPEGylated proteins.
- PK assays can be performed in multiple matrices, such as serum, plasma, cerebral spinal fluid (CSF), saliva, tissue, urine, and feces.
- We perform your PK analysis from pre-clinical and IND-enabling studies to post-market authorization.
- To successfully measure PK, we use different platforms such as ELISA (absorbance, fluorescence, and luminescence), MSD-ECL method, cell-based assays, qPCR, and RT-PCR.
Immunogenicity (ADA & NAb)
- An ADA is an antibody generated in response to a biologic drug administration.
- NAbs are a subset of ADAs that bind to the biologic (e.g. mAb) and may inhibit its efficacy.
- Detecting ADAs and NAbs is important as they can affect both the safety and efficacy of your investigational asset, ranging from lack of efficacy to severe, life-threatening side effects.
- Cerba Research has expertise with engineered proteins (pegylated and non-pegylated), peptides, ADCs, mAbs, and more. Our experience expands from design to full validation of immunogenicity methods for the detection of ADAs and NAbs.
- We have developed and validated 300+ ADA, 100+ NAb assays and cell-based activity assays and analyzed 95,000+ samples so far.
- Our method development and validation processes are customized and tailored to your specific needs and performed according to the regulatory authorities’ requirements.
- We use different platforms such as ELISA, MSD-ECL methods, cell-based assays (absorbance, fluorescence, and luminescence), qPCR and RT-PCR.

Pharmacodynamics And Biomarkers Solutions
• Biomarker analysis enables you to measure various endpoints for safety, efficacy, and exploratory use in your trial.
• Biomarkers can be performed under CAP, CLIA, GLP or, non-GLP compliant environment according to the assay’s intended use.
• We use a fit-for-purpose validation approach in accordance with your drug development stage.
• We are well-positioned to conduct simplex and multiplex biomarker panels with our MSD-ECL platfom, ELISA (absorbance, fluorescence, luminescence), gene expression by RT-qPCR, and immunophenotyping. Additional innovative capabilities through flow cytometry, next-generation sequencing and immunohistochemistry are also at your disposal.
• Biomarkers can be quantified using automated analyzers, covering clinical chemistry, hematology, coagulation, glycated hemoglobin, endocrinology, and urinalysis.
• We also offer three different peripheral blood mononuclear cell (PBMC) isolation techniques: CPT tubes, Sepmate and EasySep.

Cell-based Assay Expertise
• Cerba Research has experience in vaccines and virology by means of titer determination (plaque assays) and subtyping/hemagglutination inhibition assays.
• We also have experience in the activity of drug products and potency of drug formulations via cell-based assays.
• We rely on cell-based assays, among other techniques, for the detection of drug NAbs.
• Our scientists are highly skilled in method validation as exemplified by our more recent publications.
Want To Learn More About Our Biologics & Biosimilars Experience?
Need to talk scientist to scientist? We take a unique approach to every project with speed and agility, continuously working with your teams and proactively anticipating challenges. Reach out to us here.