What Are Hematological Malignancies?
A hematological malignancy, sometimes called a liquid tumor or blood cancer, often originates from the bone marrow, where stem cells differentiate into white blood cells, red blood cells, or platelets. Liquid tumors can be classified into three categories: leukemia, lymphoma, and multiple myeloma (1).
With increased complexity in hematological malignancy protocol design, the emergence of surrogate efficacy endpoints and biomarkers, and often rarity of disease (2), it’s important to select the right laboratory service provider for your trial. Cerba Research has a wide range of integrated specialty and safety testing options for your clinical trial needs. Get in touch to learn more.
Cerba Research Hematological Malignancy Services
Significant challenges often exist in hematological malignancy drug research and development, and complex clinical trials require a laboratory partner who provides services throughout the development spectrum. Cerba Research understands the complexities of blood cancer research and its associated treatment, diagnostics, and biomarker guidelines for various study endpoints (3). Cerba Research offers a wide range of expertise that can specifically support hematological malignancy research in this new era of cell and gene therapy (CGT) and precision medicine. We also offer end-to-end blood cancer solutions from discovery to post-market authorization for your global / regional trials.
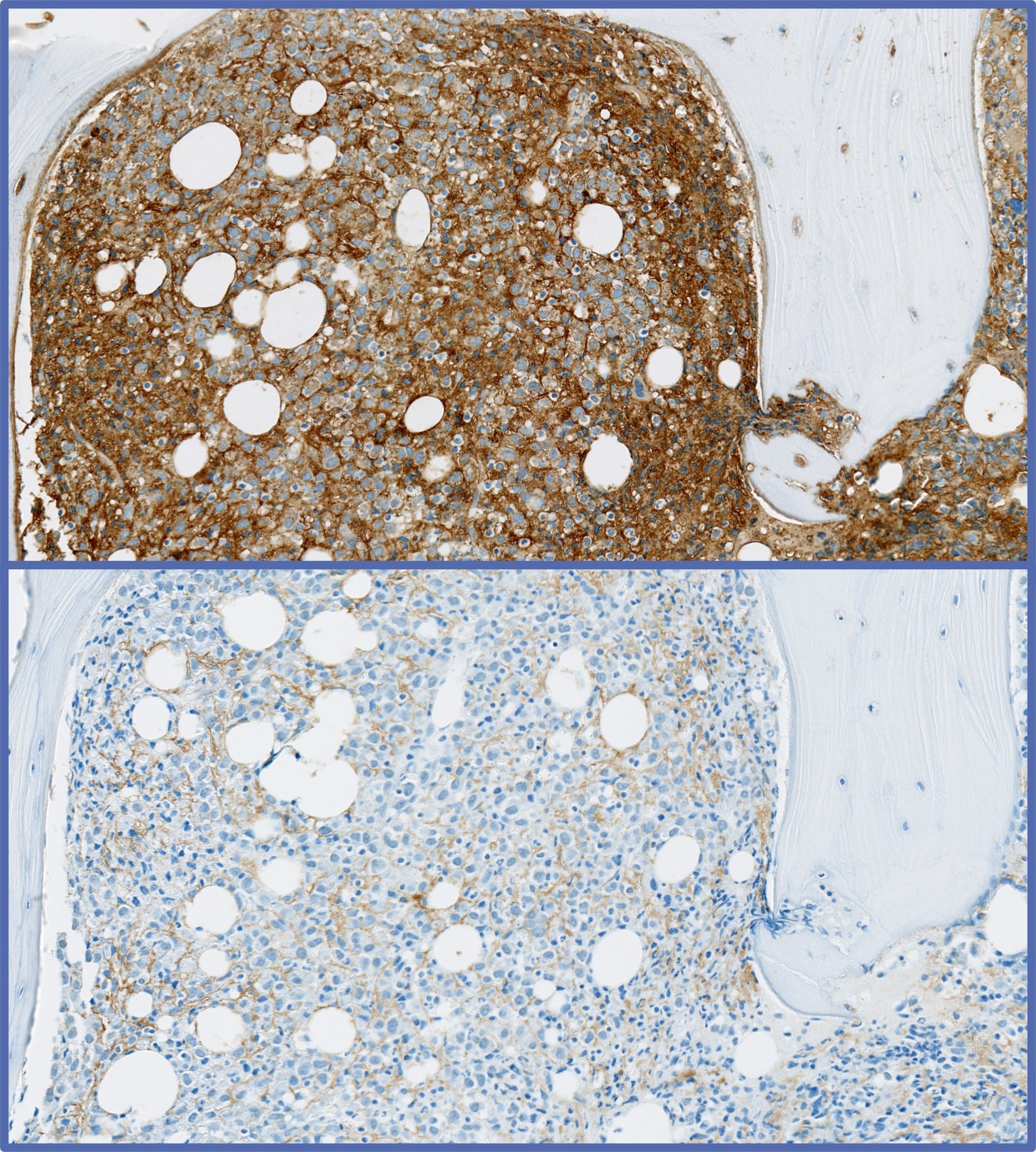
In the past 5 years, Cerba Research performed around 100 hematological malignancy trials, including first-in-human (FIH) / phase I and phase II studies. Notably, the lab has been pivotal in approving innovative drugs for indications like multiple myeloma and diffuse large B-cell lymphoma. Our team is experienced in many innovative techniques. We have a global network of laboratories that offers a wide range of capabilities, ranging from safety testing (also known as routine testing) to flow cytometry (FCM), NGS broad-panel assays, immunohistochemistry (IHC), immunoassays, immunogenicity, qPCR, ddPCR, NanoString®, and more. We also facilitate state-of-the-art technologies, subject matter expertise, specialized logistics, and operations to accelerate your blood cancer research and development.
Thanks to our expertise in assay development, validation (4), customization capabilities, robust kit building, sample management, and logistics, we have garnered about 10,500+ patients screened and 8,700+ randomized in our hematological malignancy programs since 2018.
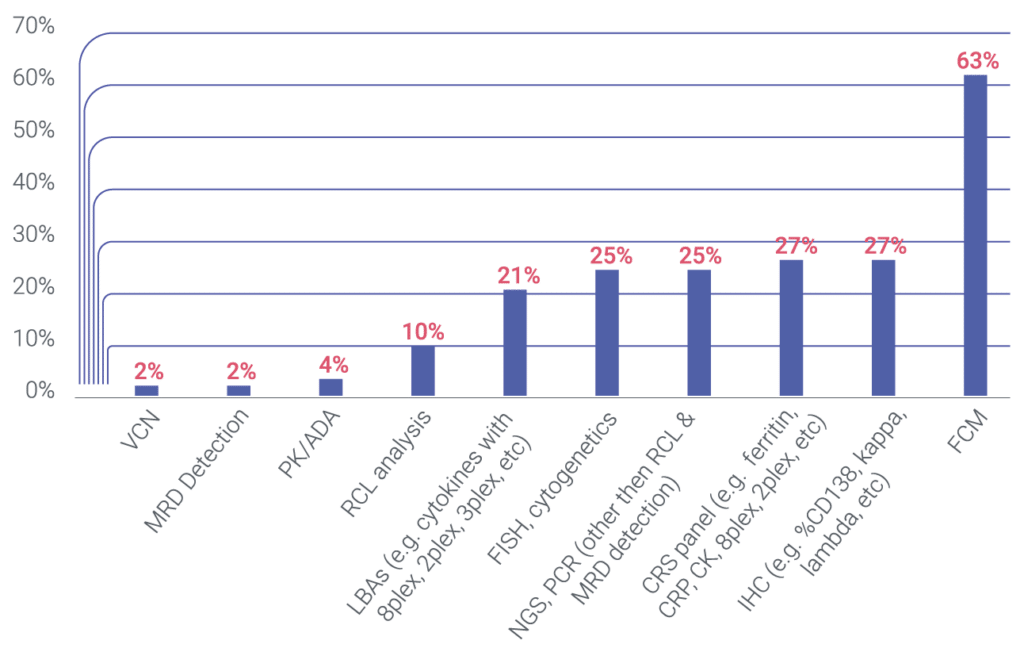
We Perform Specialty Testing In ~75% Of Our Hematological Malignancy Portfolio
We performed specialty testing in ~75% of cases within those trials. Specifically, we can perform flow cytometry (FCM), next-generation sequencing (NGS), and/or immunohistochemistry (IHC), amongst other innovative techniques. We can also perform any routine testing (aka safety testing), such as, but not limited to, coagulation, biochemistry, urinalysis, pregnancy test, COVID testing, electrophoresis, and serology, which are essential for any blood cancer trial, and patient inclusion / exclusion criteria. Additionally, Cerba Research can design and validate specialty-based assays that account for the complex tumor microenvironment of hematological malignancies.
Learn More About Our Hematological Malignancy Drug Development Capabilities
NGS, Oncopanels, broad panels, custom panels
RNAseq
Single-gene
ctDNA-based panels
ddPCR, qPCR
Whole exome / whole genome
HLA typing
TCR / BCR seq
NanoString®
SNP-Array
DNA/RNA extraction
Streck Cell-Free DNA BCT®
PaxGene®, Qiamp kits
Coagulation
Hematology
Biochemistry
Urinalysis
Pregnancy test
COVID test
Serology
Thyroid function
Multiplex cytokine profiling (37-plex)
50+ ligand binding assays
ELISA
ELLA
MSD
ELISpot
PK / ADA / Nab
FCM,
Cytek Aurora
Immunophenotyping (including intra-cell markers)
Receptor occupancy
MRD detection
CAR T cell enumeration
CAR T cell phenotyping
Intracellular cytokine detection
PBMC Isolation
BMMC Isolation
Optical genome mapping, our next-generation cytogenetics
PK / ADA / Nab
Multiplex / simplex IHC
250+ biomarkers / protocols
Full histopath service
Halo®, Visiopharm®, AIForia®
Board certified pathologists
Large biobank
Strong immuno-oncology simplex & multiplex panels
Spatial analysis of the tumor microenvironment
NanoString® GeoMx , FISH, ISH
End-to-End Services Across Your Trial Continuum
Cerba Research can execute upon every hematological malignancy trial phase, ranging from discovery / pre-clinical (data not shown) to post-market authorization trials. As such, our most decisive experience in this space is with FIH / phase I and phase II trials, which comprise 80% of our hematological malignancy portfolio. Cerba Research is active early on in oncology trials during FIH trials, where our sponsors often continue to work with us on full asset programs until registration trials and beyond. This intelligence, custom assays, and on-target protocol advice can accelerate your program to market.
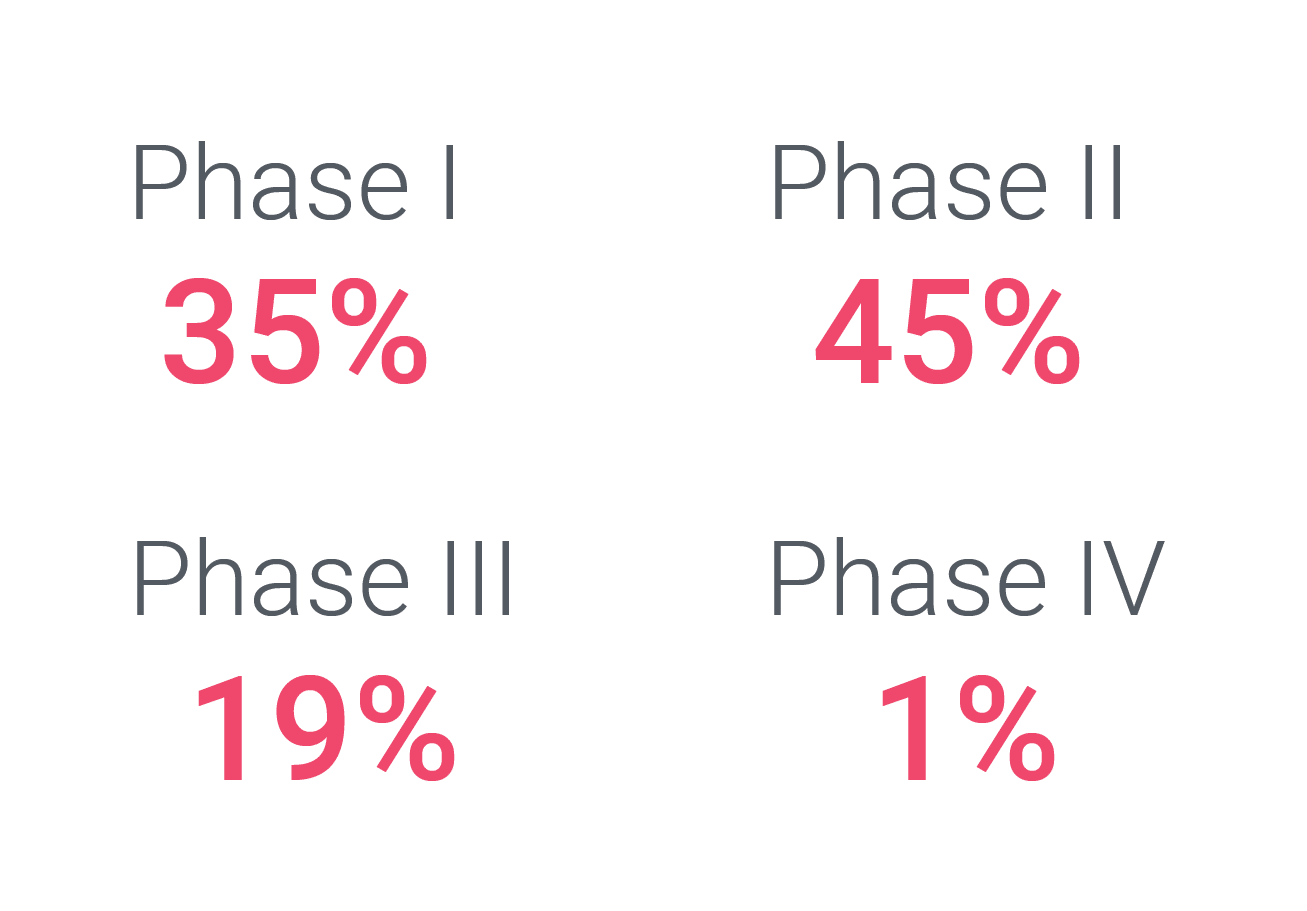
Our Areas Of Expertise In Hematological Malignancies
We have expertise in various hematological malignancies. We participated in the market authorization / expansion of 24 novel oncology therapies, which were approved for indications such as relapsed or refractory multiple myeloma and diffuse large B-cell lymphoma.
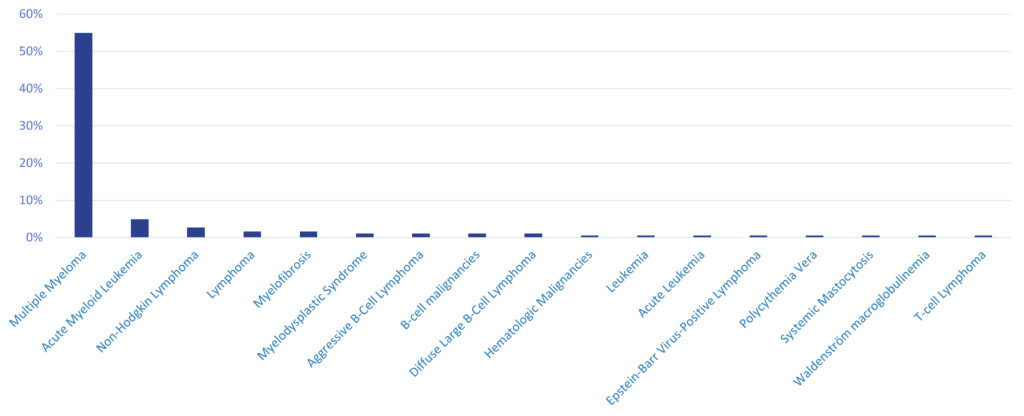
Mostly MM
Our therapy class experience is often comprised of CGTs (21% of our oncology portfolio) such as chimeric antigen receptor (CAR) T cell therapies, where the Cerba lab partners for CAR T developers who achieved FDA approvals are here.
Flow Cytometry For Hematological Malignancies
Flow cytometry (FCM) is a key tool in managing hematological malignancies. As such, immunophenotyping is often used during initial diagnosis, follow-up, and/or surveillance. Phenotypic characterization of malignant cells by FCM provides clinicians with valuable data for treatment decisions. Minimal residual disease (MRD) detection by FCM for certain hematological malignancies is life-changing for patients as MRD detection is often used as a surrogate to drug efficacy (5, 6) and is also important in select liquid tumor indications, as outlined below (3, 7).
FCM Clinical Relevance: A Key Tool In The Management Of Hematological Malignancies
| Select Liquid Tumors | Immunophenotyping | MRD Detection |
|---|---|---|
|
ALL |
X |
X* |
|
AML |
X |
X* |
|
B-Cell Lymphomas |
X |
N/A |
|
CLL |
X |
X (ERIC)*** |
|
CML |
X |
NA |
|
MM |
X |
X (Euroflow)† |
|
Myelodysplastic Syndromes |
X |
NA |
|
T-cell Lymphomas |
X |
NA |
| Select Liquid Tumors | |
|---|---|
| Immunophenotyping |
X |
| MRD Detection |
X* |
| Select Liquid Tumors | |
|---|---|
| Immunophenotyping |
X |
| MRD Detection |
X* |
| Select Liquid Tumors | |
|---|---|
| Immunophenotyping |
X |
| MRD Detection |
N/A |
| Select Liquid Tumors | |
|---|---|
| Immunophenotyping |
X |
| MRD Detection |
X (ERIC)*** |
| Select Liquid Tumors | |
|---|---|
| Immunophenotyping |
X |
| MRD Detection |
NA |
| Select Liquid Tumors | |
|---|---|
| Immunophenotyping |
X |
| MRD Detection |
X (Euroflow)† |
| Select Liquid Tumors | |
|---|---|
| Immunophenotyping |
X |
| MRD Detection |
NA |
| Select Liquid Tumors | |
|---|---|
| Immunophenotyping |
X |
| MRD Detection |
NA |
AML=acute myeloid leukemia; FCM=flow cytometry; MM=multiple myeloma; MRD=minimal residual disease; * High-sensitivity FCM with validated analysis algorithms; **FCM specifically designed to detect abnormal MRD immunophenotypes; ***6-color FCM (MRD flow) is one of two validated methods used for the detection of MRD at the level of 10-4 to 10-5

Genetics & Genomics For Hematological Malignancies
At Cerba Research, our expert team can optimize hematological malignancy trials due to our extensive experience in genomics and access to a full range of sophisticated instruments. We are also continuously verifying hematological malignancy international guidelines and adapting our existing panels accordingly (3, 6, 8).
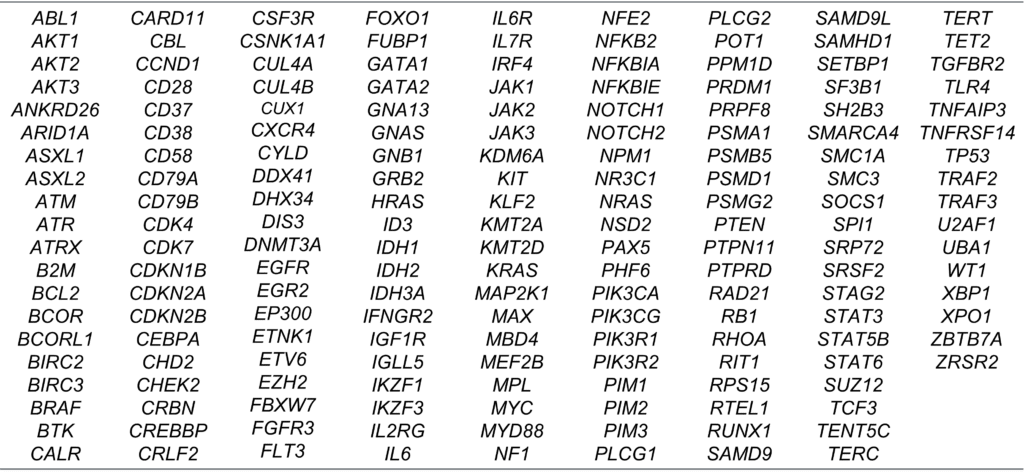
Benefit from a large offering across different hematological malignancies with our Cerba NGS extended panel (176 genes). It covers mutations with established, emerging & exploratory value across multiple myeloma, lymphoids, myeloids, myeloproliferative, myelodysplastic, acute myeloid leukemia (AML), and more. It is performed on bone marrow aspirates or whole blood.
Immunogenicity In Hematological Malignancy Trials
Our clients are supported from pre-clinical to post-market authorization with our in-depth biologics and biosimilar experience. We offer long-standing scientific expertise in pharmacokinetics (PK) and immunogenicity (antibody-drug antibodies (ADAs) and neutralizing antibodies (NAbs)) with a standardized or customized approach as per your vision.
Our focus extends from mAbs to CGTs, ADCs, and more. In addition, we are Good Laboratory Practice (GLP), the College of American Pathologists (CAP), and Clinical Laboratory Improvement Amendments (CLIA) accredited for your regulatory requirements.
References
1. Association of Cancer Care Centers™: Hematologic malignancies. URL [https://www.accc-cancer.org/home/learn/cancer-types/hematologic-malignancies].
2. Orphanet: The portal for rare diseases and orphan drugs. URL [https://www.orpha.net].
3. National Comprehensive Cancer Network®: NCCN guidelines, treatment by cancer type. URL [Treatment by Cancer Type (nccn.org)].
4. Selliah N, Nash V, Eck S, Green C, Oldaker T, Stewart J, Vitaliti A, Litwin V. Flow Cytometry Method Validation Protocols. Curr Protoc. 2023 Aug;3(8):e868. doi: 10.1002/cpz1.868. Erratum in: Curr Protoc. 2024 Jan;4(1):e988. PMID: 37606503.
5. Gaspo R, Claeys R, Sallette J. Minimal residual disease (MRD) detection trending as a primary endpoint in Cerba Research multiple myeloma (MM) trials. Presented at: EHA; June 8-11, 2023; Frankfurt, Germany.
6. ODAC: Use of Minimal Residual Disease (MRD) as an Endpoint in Multiple Myeloma Clinical Trials. URL April 12, 2024, Oncologic Drugs Advisory Committee Meeting (fda.gov).
7. Kumar S, Paiva B, Anderson KC, Durie B, Landgren O, Moreau P, Munshi N, Lonial S, Bladé J, Mateos MV, Dimopoulos M, Kastritis E, Boccadoro M, Orlowski R, Goldschmidt H, Spencer A, Hou J, Chng WJ, Usmani SZ, Zamagni E, Shimizu K, Jagannath S, Johnsen HE, Terpos E, Reiman A, Kyle RA, Sonneveld P, Richardson PG, McCarthy P, Ludwig H, Chen W, Cavo M, Harousseau JL, Lentzsch S, Hillengass J, Palumbo A, Orfao A, Rajkumar SV, Miguel JS, Avet-Loiseau H. International Myeloma Working Group consensus criteria for response and minimal residual disease assessment in multiple myeloma. Lancet Oncol. 2016 Aug;17(8):e328-e346. doi: 10.1016/S1470-2045(16)30206-6. PMID: 27511158.
8. Jennings LJ, Arcila ME, Corless C, Kamel-Reid S, Lubin IM, Pfeifer J, Temple-Smolkin RL, Voelkerding KV, Nikiforova MN. Guidelines for Validation of Next-Generation Sequencing-Based Oncology Panels: A Joint Consensus Recommendation of the Association for Molecular Pathology and College of American Pathologists. J Mol Diagn. 2017 May;19(3):341-365. doi: 10.1016/j.jmoldx.2017.01.011. Epub 2017 Mar 21. PMID: 28341590; PMCID: PMC6941185.
Discover Our Expertise In Transforming Research For Your Hematological Malignancy Treatments
We recommend starting engagement with our scientific team early, such as at the protocol design phase, for optimal results. Reach out to us here.