What Is Inflammation & Immunology?
Inflammation is an important response to potential threats and harm to our bodies. But with autoimmune disorders such as inflammatory bowel disease (IBD), psoriasis, and others, our immune system can sometimes attack us. These hurting and devastating circumstances can significantly impact a patient’s quality of life. Also, several of these conditions are sub-optimally managed by existing therapies that only offer symptom relief without looking at the root cause. For a long time, healthcare providers relied on medications such as corticosteroids as anti-inflammatories and immune suppressors. Though an important treatment option, corticosteroids come with a plethora of adverse effects.
Fortunately, science is advancing per your vision, and Cerba Research is there to focus on your I&I research. With increased complexity in I&I protocol design, the emergence of numerous biomarkers, and sometimes the rarity of disease (2), selecting the right contract research organization (CRO) for your trial is important. Don’t worry; Cerba Research has a wide range of integrated specialty and safety testing options in your existing central laboratory processes. Contact us to find out.
Cerba Research Inflammation & Immunology Services
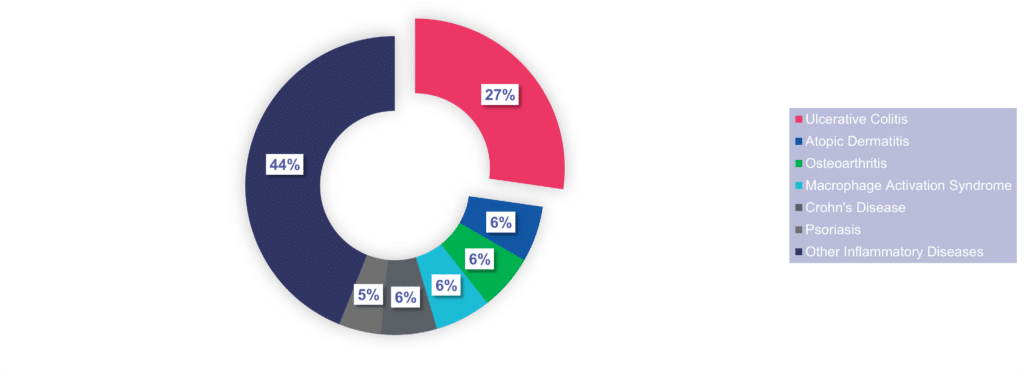
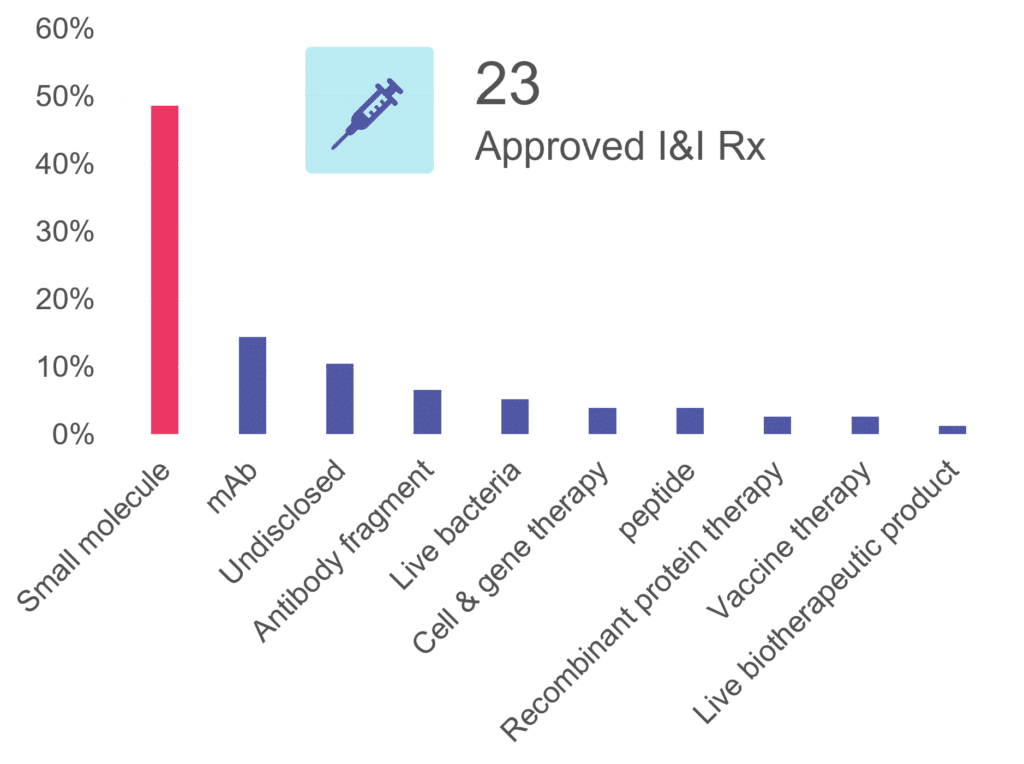
Cerba Research offers a wide range of expertise that can uniquely support I&I research and development as we offer end-to-end solutions from discovery to post-market authorization for your global/regional trials. In the past 5 years, Cerba Research performed around 80 I&I trials, including phase II and III studies. Notably, the lab has been pivotal in approving 23 innovative drugs for indications like atopic dermatitis, ulcerative colitis, Crohn’s disease, and more.
Our team is experienced in many innovative techniques. We have a global network of laboratories that offers a wide range of capabilities, ranging from safety testing (also known as routine testing) to immunoassays, immunohistochemistry (IHC), flow cytometry (FCM), NGS broad-panel assays, immunogenicity, qPCR, ddPCR, NanoString®, and more. We also facilitate state-of-the-art technologies, subject matter expertise, specialized logistics, and operations to accelerate your I&I research and development.
Thanks to our expertise in assay development, validation (2), customization capabilities, robust kit building, sample management, and logistics, we have garnered about 21,400+ patients screened and 12,500+ randomized from our I&I programs since 2018.
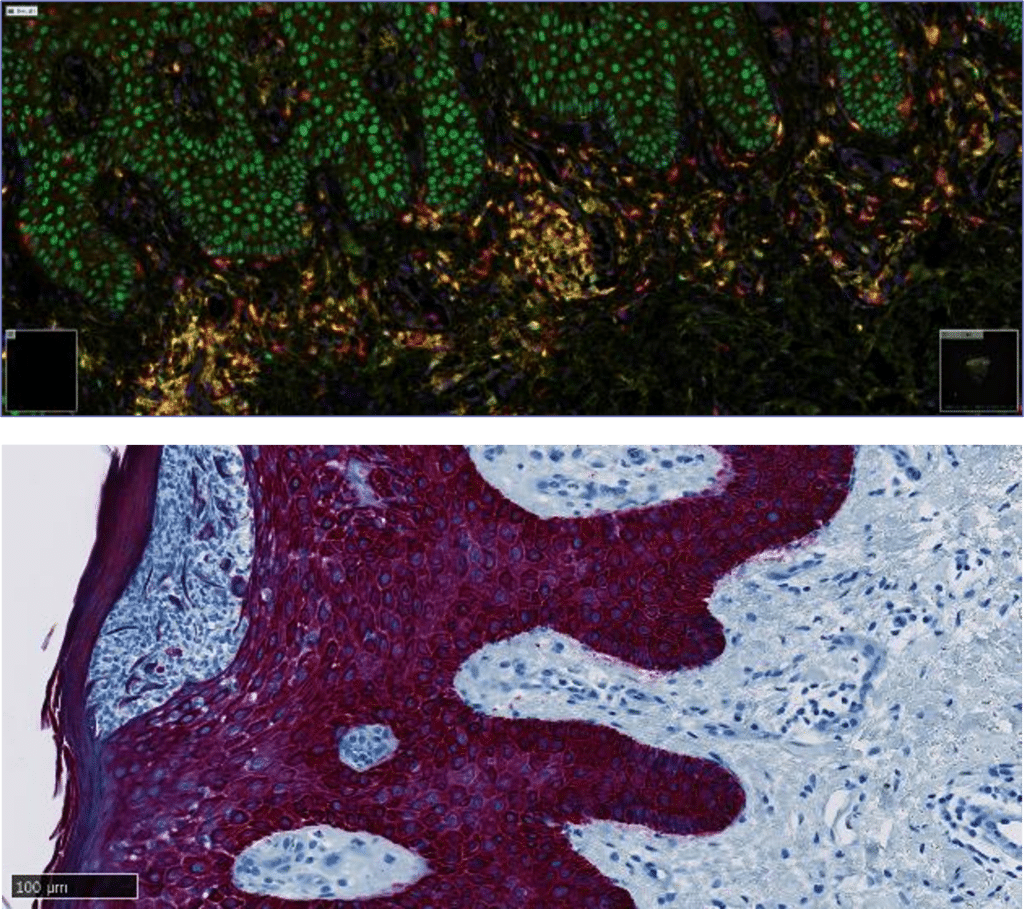
We Perform Specialty Testing In ~80% Of Our I&I Portfolio
We performed specialty testing in ~80% of cases within our I&I portfolio. Specifically, we can perform the off-the-shelf 37-plex mesoscale discovery (MSD) panel, immunohistochemistry (IHC), intracellular cytokine (ICS) detection by flow cytometry (FCM), and broad panel NGS assays, amongst other innovative techniques. We can also perform any routine testing (aka safety testing), such as, but not limited to, coagulation, biochemistry, urinalysis, pregnancy test, COVID testing, and serology, which are essential for any I&I trial and patient inclusion / exclusion criteria. Do you need a new test to be validated? We can help design and validate your specialty-based assays with our fit-for-purpose approach.
Learn More About Our I&I Drug Development Capabilities
Validated assays to detect most kinds of cytokines and chemokines with our 37-plex off-the-shelf panel. 50+ ELISA-based assays.
BD FACS Canto & Lyric, Cytek Aurora instruments, for intracellular cytokine detection (ICD), follow up on immune cells status and more.
PBMC, BMMC, CD138+, … PBMC processing in 25+ countries with 45+ processing labs and growing …
NGS, WGS, WES, single gene, RNA-seq, TCR/BCR seq, qPCR, ddPCR. Custom Panels and already existing broad-panel assays for I&I diseases.
Detecting and quantifying gene/viral genome, including follow-up for cell and gene therapy. Also including, but not limited to, RCR, RCL and VCN.
FISH, ISH, NanoString®GeoMx & IHC with 250+ IHC protocols available for analysis.
End-to-End Services Across Your Trial Continuum
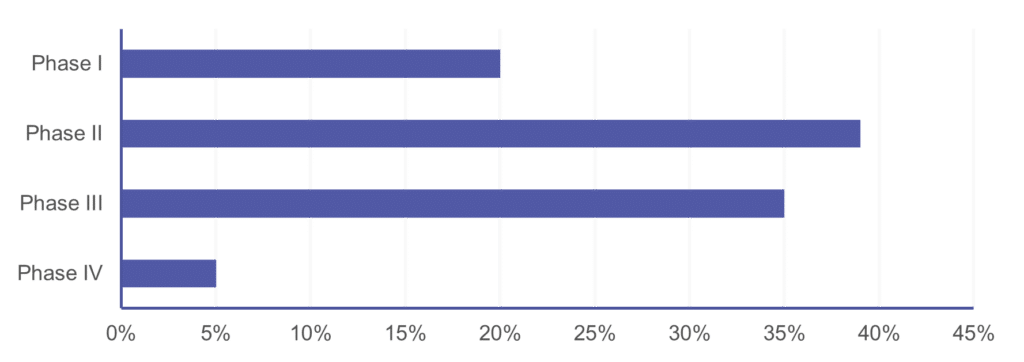
Cerba Research can execute upon every I&I trial phase, ranging from discovery / pre-clinical (data not shown) to post-market authorization trials. As such, our most decisive experience in this space is with phase II and III trials, which comprise around 75% of our I&I portfolio. Cerba Research is also active early on in I&I trials during first-in-human trials, where our sponsors often continue to work with us on full asset programs until registration trials and beyond. This intelligence, custom assays, and on-target protocol advice can accelerate your program to market.
We have expertise in various I&I indications. We participated in the approval of 23 novel I&I therapies that were marketed for indications such as atopic dermatitis, ulcerative colitis, and Crohn’s disease.
Of mention, our therapy class experience is often comprised of small molecules (about half of our I&I portfolio) but also monoclonal antibodies (mAb), cell and gene therapies (CGT), and peptides.
37plex Off-the-shelf MSD Panel
Many diseases are characterized by inflammatory processes. Mesoscale discovery (MSD) can be utilized for analyzing biomarkers, especially most kinds of cytokines, chemokines, and pro-inflammatory markers, with our 37-plex off-the-shelf panel. Our panel combines 5 multiplex panels with high sensitivity and precision. Moreover, the multiplex setup offers excellent flexibility and is customizable according to your I&I disease trial requirements while retaining comparability and consistency over different projects. The preferred matrix is EDTA plasma or serum.
Our Off-the-shelf 37-plex MSD Panel
| Proinflammatory | Chemokine | Cytokine | Angiogenesis | Vascular |
|---|---|---|---|---|
|
TNF-α
|
Eotaxin |
GM-CSF |
VEGF-A |
SAA |
|
IFN-γ |
Eotaxin-3 |
IL-5 |
VEGF-D |
CRP |
|
IL-1ß |
MIP-1α |
IL-7 |
Tie-2 |
VAM-1 |
|
IL-2 |
MIP-1ß |
IL-12/IL23p40 |
Flt-1 |
ICAM-1 |
|
IL-4 |
IP-10 |
IL-15 |
PlGF |
|
|
IL-6 |
MCP-1 |
IL-16 |
bFGF |
|
|
IL-8 |
MCP-4 |
IL-17A |
||
|
IL-10 |
MDC |
TNF-ß |
||
|
IL-13 |
| Proinflammatory | |
|---|---|
| Chemokine |
Eotaxin |
| Cytokine |
GM-CSF |
| Angiogenesis |
VEGF-A |
| Vascular |
SAA |
| Proinflammatory | |
|---|---|
| Chemokine |
Eotaxin-3 |
| Cytokine |
IL-5 |
| Angiogenesis |
VEGF-D |
| Vascular |
CRP |
| Proinflammatory | |
|---|---|
| Chemokine |
MIP-1α |
| Cytokine |
IL-7 |
| Angiogenesis |
Tie-2 |
| Vascular |
VAM-1 |
| Proinflammatory | |
|---|---|
| Chemokine |
MIP-1ß |
| Cytokine |
IL-12/IL23p40 |
| Angiogenesis |
Flt-1 |
| Vascular |
ICAM-1 |
| Proinflammatory | |
|---|---|
| Chemokine |
IP-10 |
| Cytokine |
IL-15 |
| Angiogenesis |
PlGF |
| Vascular | |
| Proinflammatory | |
|---|---|
| Chemokine |
MCP-1 |
| Cytokine |
IL-16 |
| Angiogenesis |
bFGF |
| Vascular | |
| Proinflammatory | |
|---|---|
| Chemokine |
MCP-4 |
| Cytokine |
IL-17A |
| Angiogenesis | |
| Vascular | |
| Proinflammatory | |
|---|---|
| Chemokine |
MDC |
| Cytokine |
TNF-ß |
| Angiogenesis | |
| Vascular | |
| Proinflammatory | |
|---|---|
| Chemokine | |
| Cytokine | |
| Angiogenesis | |
| Vascular | |
Histopathology And Immunohistochemistry
At Cerba Research, we are committed to delivering gold-standard tissue sourcing, processing, and analysis services for your I&I trial. Cerba Research offers over 250 IHC biomarkers off-the-shelf, and our catalog covers a wide range of indications, such as atopic dermatitis and IBD, among others.
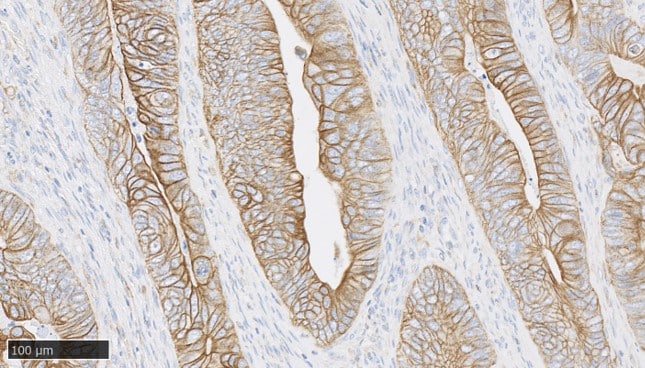
Spatial OMICS Solution (NanoString®)
Conducting I&I studies often requires simultaneous multiparameter assessment of the clinical specimen, such as histological material. Besides standard immunological staining, the development of novel technological solutions such as NanoString® allows us to analyze tissue in a previously unapparelled way, simultaneously assessing not only the presence and location of the antigen of interest but also quantifying levels of gene and protein expression (3).
Intracellular Cytokine Detection by Flow Cytometry
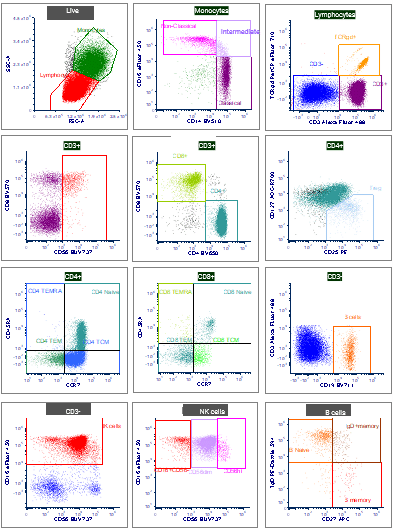
Flow cytometry (FCM) became an indispensable tool in immunology, cancer research, and clinical diagnostics, helping researchers deeply understand the immune system and perform cellular immunophenotyping with remarkable precision. FCM provides in-depth immunizations of immune cells, including activation and exhaustion status, and functional characterization, such as proliferation and cytokine production. Therefore, Cerba Research can thoroughly assess functional T cells with intracellular cytokine staining by FCM to evaluate your immune modulators.
Immunophenotyping By Flow Cytometry
Immune monitoring of patients enrolled in clinical drug development trials is pivotal to supporting the evaluation of drug effect, safety, and toxicity. Advancements in FCM technology allow us to expand the assays to high-parameter panels. Spectral FCM has exciting potential for deep characterizing immune subsets for patient samples from global clinical trials. Developing high-parameter panels with spectral FCM allows a deeper characterization of patient samples in response to immune therapies (4). Reach out to learn more.
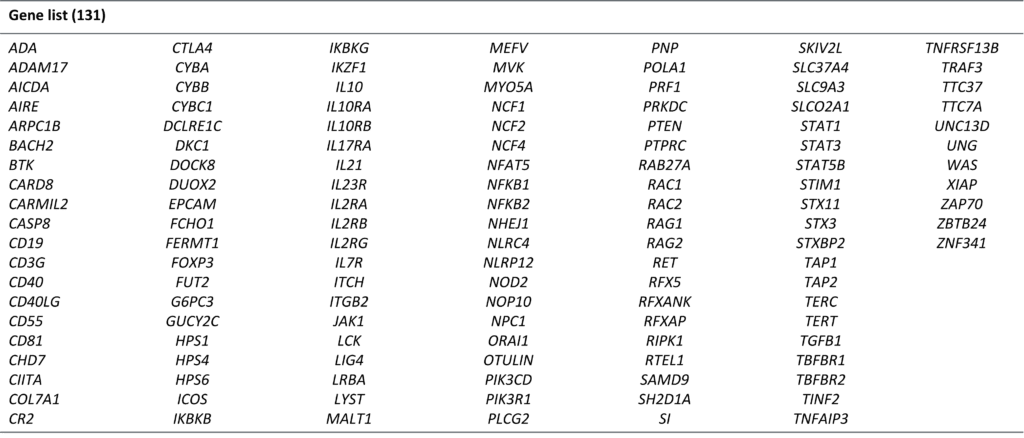
Genetics & Genomics For I&I
At Cerba Research, our expert team can optimize I&I trials due to our extensive experience in genomics and access to a full range of sophisticated instruments. The targeted gene sequencing approach is a useful tool when looking for specific gene mutations or determining the presence of a gene of interest in your sample. Specifically designed panels contain selected genes with known or suspected associations with autoimmune diseases. Multiple genes can be assessed across many samples in parallel, saving time and reducing costs associated with running multiple separate assays. Benefit from a large NGS offering across different I&I indications with our Cerba IBD comprehensive NGS panel (131 genes) and more.
Immune Repertoire Profiling
The adaptive component of the immune system, also known as acquired immunity, is composed of a highly specialized and diverse population of B and T lymphocytes poised to recognize and eliminate invading pathogens or other sources of unknown antigens.
Applying NGS technologies enables us to profile these populations with high resolution. Whether we are profiling the B or T cell repertoire, parameters such as clonality, diversity, and richness enhance our ability to harness the potential of the clones of interest for therapeutic, diagnostic, or research use.
Immunogenicity For Your I&I Trials
With our in-depth biologics and biosimilar experience, we support our clients from pre-clinical to post-market authorization. We offer long-standing scientific expertise in pharmacokinetics (PK) and immunogenicity (anti-drug antibodies (ADAs) and neutralizing antibodies (NAbs)) with a standardized or customized approach, as per your vision.
Did you know that Cerba Research Canada was instrumental in the PK and immunogenicity validation and implementation of interferon beta-1a, a protein approved globally for multiple sclerosis (5)? We were also instrumental in the PK and immunogenicity implementation of natalizumab and adalimumab, mAbs approved for various I&I conditions (6, 7).
Our focus extends from mAbs to CGTs, antibody-drug conjugates, and more. In addition, we are Good Laboratory Practice (GLP), the College of American Pathologists (CAP), and Clinical Laboratory Improvement Amendments (CLIA) accredited for your regulatory requirements.
References
1. Orphanet: The portal for rare diseases and orphan drugs. URL [https://www.orpha.net].
2. Selliah N, Nash V, Eck S, Green C, Oldaker T, Stewart J, Vitaliti A, Litwin V. Flow Cytometry Method Validation Protocols. Curr Protoc. 2023 Aug;3(8):e868. doi: 10.1002/cpz1.868. Erratum in: Curr Protoc. 2024 Jan;4(1):e988. PMID: 37606503.
3. Robles-Remacho A, Sanchez-Martin RM, Diaz-Mochon JJ. Spatial Transcriptomics: Emerging Technologies in Tissue Gene Expression Profiling. Anal Chem. 2023 Oct 24;95(42):15450-15460. doi: 10.1021/acs.analchem.3c02029. Epub 2023 Oct 10. PMID: 37814884; PMCID: PMC10603609.
4. Feyzâ Matisli, Jan Spitaels, Veronica Nash, Amber Baele, Leen Catrysse, Silke De Waele, Miet De Baere, Nithianandan Selliah. Validation of a Flow Cytometry Assay on Cytek® Aurora to Monitor Immune Cells in Peripheral Whole Blood for Clinical Trials. Presented at: European Society for Clinical Cell Analysis (ESCCA); 27-30 September 2023; Utrecht, Netherlands.
5. Rebif® (interferon beta-1a): Highlights of prescribing information. URL [REBIF (interferon beta-1a) Prescribing Information (emdserono.com)].
6. Tysabri® (natalizumab): Highlights of prescribing information. URL [TYSABRI Label (fda.gov)].
7. Humira® (adalimumab): Highlights of prescribing information. URL [Humira (adalimumab) label (fda.gov)].
Discover Our Expertise In Transforming Research For Your I&I Innovation

We recommend starting engagement with our scientific team early, such as at the protocol design phase, for optimal results. Reach out to us here.
Contact Us