What Is Oncology?
Oncology is a branch of medicine that specializes in the diagnosis and treatment of cancers. It includes medical oncology, such as chemotherapy, hormonal therapy, targeted therapies, immuno-oncology (I/O), cell and gene therapies (CGTs), and other drugs. It also includes radiation therapy, which uses radiation to attempt to eradicate a tumour, and surgical intervention, which uses surgery and other procedures to treat cancers (1).
With increased complexity in oncology protocol design, endpoints, and time sensitivity due to end-of-life clinical trial patients (2), Cerba Research provides an adaptive oncology team that has access to the latest science and technology to optimize the outcome of your trial(s). Precision medicine development requires a flexible and agile specialty laboratory partner who can adjust as the protocol does, ensuring endpoints are captured appropriately and enabling studies to move forward with the right data at the right time. As such, Cerba Research can provide a wide range of integrated specialty and safety testing to your existing central laboratory processes.
Cerba Research’s Oncology Services
There are often significant challenges to oncology drug research and development, and complex oncology clinical trials require a laboratory partner that provides services throughout the development spectrum. Cerba Research understands the complexities of cancer research and its associated treatment guidelines (3) and biomarkers that are used for various study endpoints (primary, secondary, and exploratory).
Cerba Research offers a wide range of expertise and capabilities that can specifically support oncology research in this new era of precision medicine, biomarkers, next-generation sequencing (NGS) broad-panel assays, and liquid biopsies (also known as circulating tumor DNA (ctDNA)). We also offer end-to-end oncology solutions from discovery to post-market authorization for your global/regional trials. This includes protocol review to ensure the right innovative techniques are applied at the right time, even when prior proposals are being submitted.
Cerba Research has significant experience in oncology clinical research, across many solid tumors and hematological malignancies. We have a global network of laboratories that offers a wide range of capabilities, ranging from safety testing (also known as routine testing) to NGS broad-panel assays, immunohistochemistry (IHC), flow cytometry (FCM), immunoassays, immunogenicity, qPCR, ddPCR, NanoString®, and more.
Developing oncology innovative therapies requires increased access to expertise, knowledge, data, and insights to bring the right patient to the proper treatment and/or trial at the right time. We facilitate state-of-the-art technologies, subject matter expertise, specialized logistics, and operations to accelerate your oncology research and development.
Oncology Is A Priority In The Clinical Trial Landscape
Oncology research and development is a priority for drug developers, patients, their families, regulators, non-profit organizations, and healthcare providers. However, our sponsors face significant challenges when trying to advance cancer research and innovative treatments because of the following:
- Cancer is one of the most complex diseases known to mankind.
- Cancer is not just one cancer but more than 100 cancer types (and many more subtypes) affecting people in different ways (3).
- Some cancers are difficult to diagnose, especially rare neoplasms.
- Cancer patients are often fragile and geographically dispersed.
- NGS broad-panel assays and other innovative techniques are not readily available on a global scale (4).
- Lack of consistency for safety testing results at the site level when a central laboratory is not utilized, especially for difficult to diagnose malignancies such as multiple myeloma.
Cerba Research is uniquely positioned to support your oncology trial with a wide range of in-house laboratory solutions depending on your specific indication. Thanks to our expertise in assay development, validation (5), customization capabilities, robust kit building, sample management, and logistics, we have garnered 29,400+ patients screened and 23,300+ randomized within our oncology clinical programs since 2018.
We Perform Specialty Testing In ~75% Of Our Oncology Portfolio
We have had ~200 oncology trials since 2018 and growing. We perform specialty testing in ~75% of cases within those trials. Specifically, we can perform immunohistochemistry (IHC), flow cytometry (FCM), and/or NGS, amongst other innovative techniques. We can also perform any routine testing (aka safety testing), such as, but not limited to, coagulation, biochemistry, urinalysis, pregnancy test, COVID testing and serology, which are essential for any oncology trial and patient inclusion / exclusion criteria. We can also help design and validate specialty-based assays that account for the complex tumor microenvironment of both solid tumors and hematological malignancies.
Learn More About Our Oncology Drug Development Capabilities
End-to-End Services Across Your Trial Continuum
Cerba Research can execute upon every oncology trial phase, ranging from discovery/pre-clinical (data not shown) to post-market authorization trials. As such, our most decisive experience in oncology is with first-in-human (FIH) phase I and phase II trials, which comprise close to 80% of our oncology portfolio. We engage early on in oncology trials where our sponsors often continue to work with us on full asset programs until registration trials and beyond. This intelligence, along with custom assays and on-target protocol advice, can accelerate your program to market.

Cerba Research has supported biopharma and pharmaceutical companies in developing oncology therapies. Our heritage in specialty labs and our experience in central lab services enable us to develop research techniques to underpin the next generation of clinical trials. A new kind of research where diagnostics are driven by clinical data and insights, supported by specific therapeutic expertise.
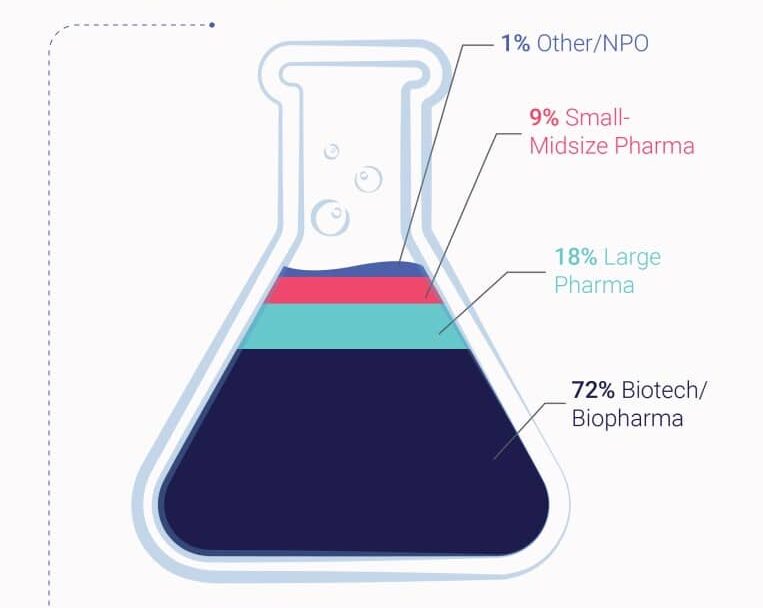
Our Areas Of Expertise In Oncology
We have expertise in both solid tumors and hematological malignancies (almost half and half). We participated in the approval of 24 novel oncology therapies marketed for various indications, such as, but not limited to, relapse and/or refractory multiple myeloma, diffuse large B-cell lymphoma, metastatic breast cancer, neuroblastoma, and non-small cell lung cancer (NSCLC) amongst other indications.
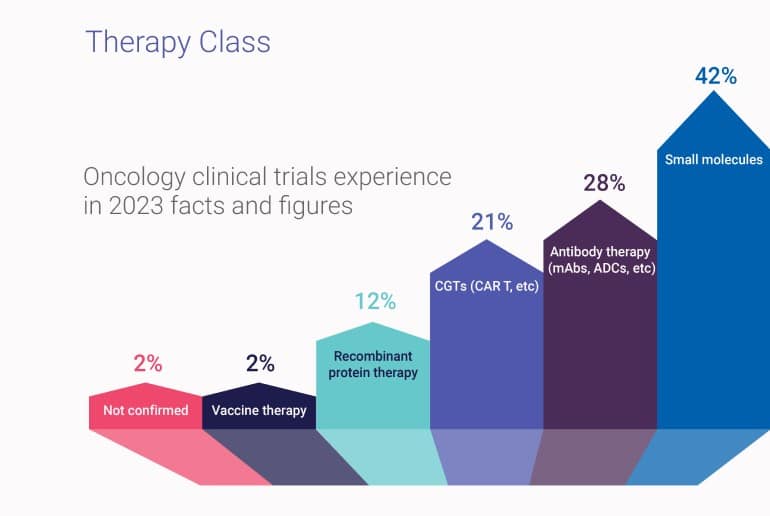
Our therapy class experience is highest with small molecules (42% of our oncology trials), followed by antibody-specific therapies (28%) such as monoclonal antibodies (mAbs), antibody-drug conjugates (ADCs), and bispecific antibodies. We also have rich experience with complex CGTs (21%), mostly with chimeric antigen receptor-T cell therapies (CAR T) that are often evaluated in hematological malignancies.
Immunogenicity In Oncology
Our clients are supported from pre-clinical to post-market authorization with our in-depth biologics and biosimilars experience. We offer long-standing scientific expertise in pharmacokinetics (PK) and immunogenicity (antibody-drug antibodies (ADAs) and neutralizing antibodies (NAbs)) with a standardized or a customized approach as per your vision.
Did you know that Cerba Research Canada was instrumental in the PK and immunogenicity validation and implementation of bevacizumab? A mAb approved globally for various solid tumours, such as metastatic colorectal cancer, advanced or metastatic NSCLC, and recurrent glioblastoma among other tumour types (6). We were also instrumental in the PK and immunogenicity implementation of pegfilgrastim, a recombinant protein marketed for supportive care oncology (e.g. chemotherapy-induced febrile neutropenia (7). Our focus extends from mAbs to CGTs, ADCs and more. In addition, we are good laboratory practice (GLP), college of American pathologists (CAP), clinical laboratory improvement amendments (CLIA) accredited for your regulatory requirements.
References
1. National Cancer Institute: NCI dictionary of cancer terms. Definition of oncology. URL [NCI Dictionary of Cancer Terms – NCI].
2. Getz K, Smith Z, Kravet M. Protocol Design and Performance Benchmarks by Phase and by Oncology and Rare Disease Subgroups. Ther Innov Regul Sci. 2023 Jan;57(1):49-56. doi: 10.1007/s43441-022-00438-5. Epub 2022 Aug 12. PMID: 35960455; PMCID: PMC9373886.
3. National Comprehensive Cancer Network®: NCCN guidelines, treatment by cancer type. URL [Treatment by Cancer Type (nccn.org)].
4. Bebb DG, Banerji S, Blais N, Desmeules P, Gill S, Grin A, Feilotter H, Hansen AR, Hyrcza M, Krzyzanowska M, Melosky B, Noujaim J, Purgina B, Ruether D, Simmons CE, Soulieres D, Torlakovic EE, Tsao MS. Canadian Consensus for Biomarker Testing and Treatment of TRK Fusion Cancer in Adults. Curr Oncol. 2021 Jan 15;28(1):523-548. doi: 10.3390/curroncol28010053. PMID: 33467570; PMCID: PMC7903287.
5. Selliah N, Nash V, Eck S, Green C, Oldaker T, Stewart J, Vitaliti A, Litwin V. Flow Cytometry Method Validation Protocols. Curr Protoc. 2023 Aug;3(8):e868. doi: 10.1002/cpz1.868. Erratum in: Curr Protoc. 2024 Jan;4(1):e988. PMID: 37606503.
6. Avastin® (bevacizumab): Highlights of prescribing information. URL [avastin_prescribing.pdf (gene.com)].
7. Neulasta® (pegfilgrastim): Highlights of prescribing information. URL [NEULASTA (pegfilgrastim) Label (fda.gov)].
Discover Our Expertise In Transforming Research For Your Oncology Therapies
We recommend starting engagement with our scientific team early, such as at the protocol design phase, for optimal results. Reach out to us here.