Fact Sheet – Unveiling the Oncology Journey: Highlighting Cutting-Edge Capabilities
Cerba Research has conducted nearly 200 oncology trials within the last 5…
Read more
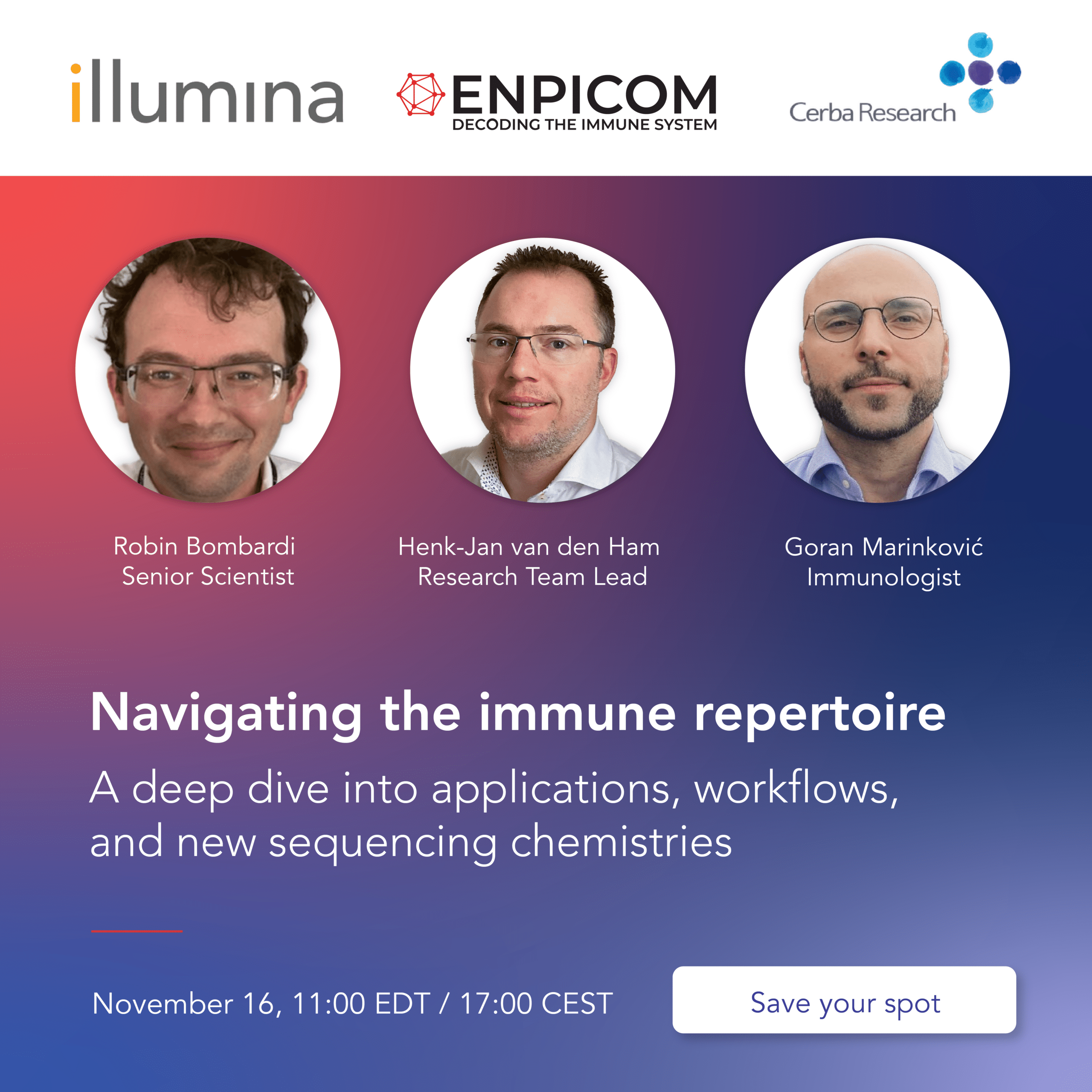
Webinar – Webinar – Advancing CAR T-Cell Therapies with Clinical Trial Customization
Our experts Goran Marinkovic, PhD, Immunologist, Nele Langenaken, General Manager, Dr. Nithianandan Selliah,…
Read more

Fact Sheet – Virology Center of Excellence: Illuminating Insights Into Infectious Diseases
Experience the unparalleled activity of Cerba Research’s Infectious Diseases (ID) therapy sector,…
Read more

Fact Sheet – Unlocking The Potential Of Rare Disease Drug Development
Discover the extraordinary world of rare diseases, where uniqueness defines the landscape.…
Read more

Webinar – Advancing CAR T-Cell Therapies with Clinical Trial Customization
We are excited to announce our webinar in collaboration with Xtalks – Advancing CAR…
Read more

Together, we can shape the future of your clinical development. Find out more about how we can support your research.
Contact Us