Supporting Your Biomarker Journey
Cerba Research has extensive experience in developing IHC protocols from preclinical models to validating laboratory-developed tests for clinical trials. Our science-driven histology experts can provide complete and efficient IHC assay development, optimization, transfer, and fit-for-purpose validation. This allows you to take your study to its goal, whether it calls for simplex or multiplex immunohistochemistry protocol(s).
- Cerba Research offers over 250 human IHC biomarkers off-the-shelf, as well as a diverse list of protocols for animal models
- Our catalog covers a wide range of areas of research, including immuno-oncology, dermatology, immunology, neurosciences, and metabolic disorders
- Our histology scientific team has extensive experience developing and validating new immunohistochemistry assays
- With our large choice of IVD (Benchmark Ultra, Dako Omnis, Leica Bond III) and RUO (Discovery Ultra, Leica Bond Rx) platforms, and strong knowledge of reagents and optimization methods, we offer standard development workflows for IHC and multiplex IHC with a high success rate
- With a growing biobank of over 3,000 blocks (frozen and FFPE, animal and human, healthy and diseased), a network of over 25 government-authorized hospitals, and providers of custom sourcing, Cerba Research can accelerate your new project
- With capabilities for custom cell controls and tumor micro-arrays, Cerba Research can offer the most trusted and cost-efficient IHC protocol development and validation approach for anatomic pathology and tissue analysis
- Each validation program is managed as a collaborative interaction between our scientists and the sponsor, with high-quality validation plans and reports, as well as frequent updates on progression
- The results for general validation parameters are supported by our pathologists, image analysis, and statistics teams
- Complementary techniques for orthogonal validation are available
- The Cerba Research team can offer the most appropriate approach for obtaining results from IHC sections; with the ability to scan both brightfield and fluorescence, an extensive and diverse network of clinical and veterinary pathologists, and software solutions for image analysis that include deep learning.
- Our three histology laboratories, located in the USA (New York), Europe (Montpellier, France), and Asia (Taipei, Taiwan) work together to ensure optimal results regardless of where the sample is run
- Standard operating procedures, quality controls, and platforms are key comparable factors for full transparency
Discover The Art of Histopathology book
Our IHC expert scientist team will consult and work with you on your specific needs.
Flexible in our approach and delivery, we provide timely and cost-effective solutions to meet your clinical and commercial objectives.
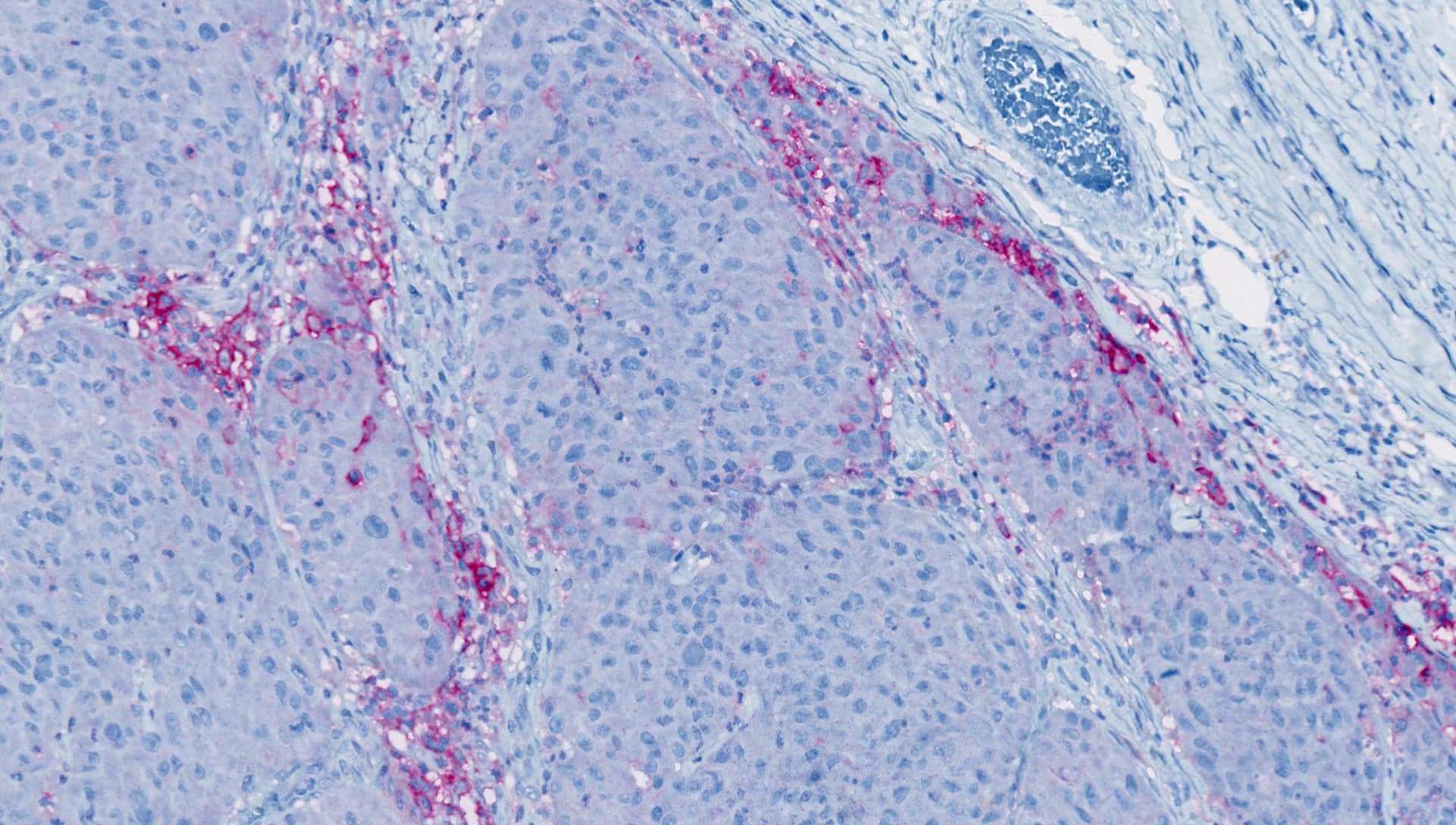
A Biomarker Journey in Immuno-oncology Utilizing IHC
With immunohistochemistry (IHC) technology, biomarkers can be identified in tumor biopsies. IHC provides the possibility to better understand biomarker mechanisms in the tumor’s environment.
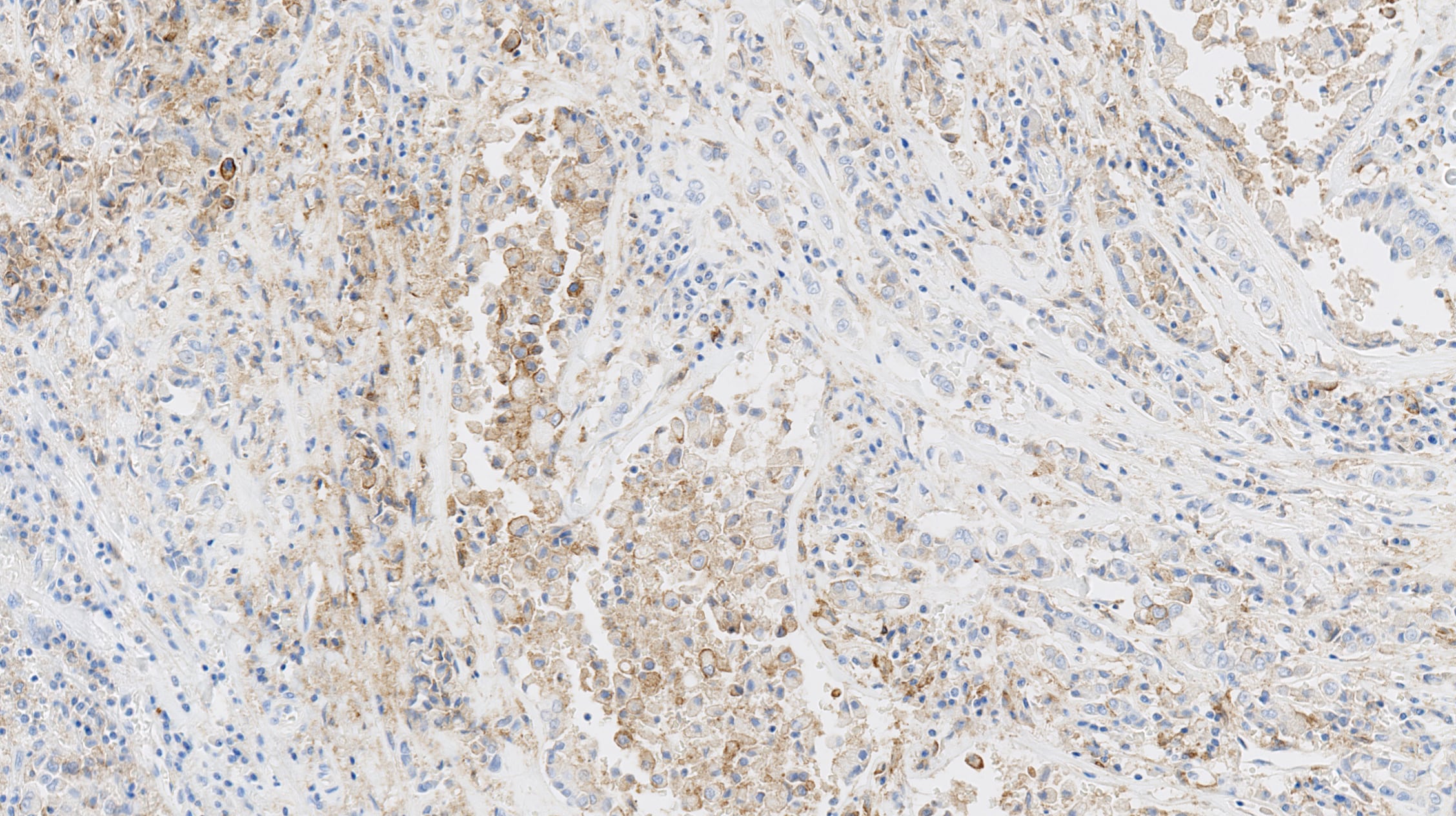
Brochure – Cerba Research IHC Biomarkers

Reach out to our experts to see how we can help you with your Immunohistochemistry needs!
Contact Us
